Doxorubicin (DOX) is a potent chemotherapeutic agent and has toxic effects on various organs, including the liver. In the current study, we aimed to investigate the effects of bone-marrow-derived mesenchymal stem cell (BM-MSC) administration on DOX-induced hepatotoxicity in rats. 24 Wistar-albino rats were divided into three groups: Control, DOX, and DOX+MSC. DOX (20 mg/kg) was administered to the DOX group. In the DOX + MSC group, BM-MSCs (2 × 106 ) were given through the tail vein following DOX administration. DOX administration led to significant structural liver injury. Besides this, oxidative balance in the liver was impaired following DOX administration. DOX administration also led to an increase in apoptotic cell death in the liver. Structural and oxidative changes were significantly alleviated with the administration of BM-MSCs. Furthermore, BM-MSC administration suppressed excessive apoptotic cell death. Our findings revealed that BM-MSC administration may alleviate DOX-induced liver injury via improving the oxidative status and limiting apoptotic cell death in the liver tissue.
© 2022 Wiley Periodicals LLC.
Product Citations: 66
Effects of bone marrow-derived mesenchymal stem cells on doxorubicin-induced liver injury in rats.
In Journal of Biochemical and Molecular Toxicology on 1 April 2022 by Celik Samanci, T., Gökçimen, A., et al.
-
FC/FACS
-
Rattus norvegicus (Rat)
-
Biochemistry and Molecular biology
-
Stem Cells and Developmental Biology
In Scientific Reports on 7 March 2022 by Nasiri, N., Hosseini, S., et al.
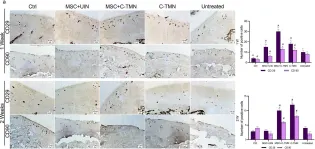
Mesenchymal stem cells (MSCs) are at the forefront of research for a wide range of diseases, including osteoarthritis (OA). Despite having attracted the attention of orthopedists, current MSC therapy techniques are limited by poor MSC implantation in tissue defects and lack of lateral tissue integration, which has restricted the efficacy of cell therapy to alleviate OA symptoms only. Here, we developed targeted MSC therapy for OA cartilage using a cell-tissue matchmaking nanoconstruct (C-TMN). C-TMN, as an MSC vehicle, consists of a central iron oxide nanoparticle armed with two types of antibodies, one directed at the MSC surface and the other against articular cartilage. We treated rat OA articular cartilage with intra-articular injections of C-TMN with and without exogenous MSCs. We observed substantial improvements in both symptomatic and radiographic OA caused by C-TMN, which was independent of exogenous MSCs. This new approach could predict a promising future for OA management.
© 2022. The Author(s).
-
IHC
-
Rattus norvegicus (Rat)
-
Stem Cells and Developmental Biology
Autophagy induction during stem cell activation plays a key role in salivary gland self-renewal.
In Autophagy on 1 February 2022 by Orhon, I., Rocchi, C., et al.
Relatively quiescent tissues like salivary glands (SGs) respond to stimuli such as injury to expand, replace and regenerate. Resident stem/progenitor cells are key in this process because, upon activation, they possess the ability to self-renew. Macroautophagy/autophagy contributes to and regulates differentiation in adult tissues, but an important question is whether this pathway promotes stem cell self-renewal in tissues. We took advantage of a 3D organoid system that allows assessing the self-renewal of mouse SGs stem cells (SGSCs). We found that autophagy in dormant SGSCs has slower flux than self-renewing SGSCs. Importantly, autophagy enhancement upon SGSCs activation is a self-renewal feature in 3D organoid cultures and SGs regenerating in vivo. Accordingly, autophagy ablation in SGSCs inhibits self-renewal whereas pharmacological stimulation promotes self-renewal of mouse and human SGSCs. Thus, autophagy is a key pathway for self-renewal activation in low proliferative adult tissues, and its pharmacological manipulation has the potential to promote tissue regeneration.
-
Cell Biology
-
Stem Cells and Developmental Biology
In Stem Cell Research & Therapy on 15 July 2021 by Zhu, L., Feng, Z., et al.
Accumulating evidence suggests that enhanced adipose tissue macrophages (ATMs) are associated with metabolic disorders in obesity and type 2 diabetes. However, therapeutic persistence and reduced homing stem cell function following cell delivery remains a critical hurdle for the clinical translation of stem cells in current approaches.
We demonstrate that the effect of a combined application of photoactivation and adipose-derived stem cells (ASCs) using transplantation into visceral epididymal adipose tissue (EAT) in obesity. Cultured ASCs were derived from subcutaneous white adipose tissue isolated from mice fed a normal diet (ND).
In diet-induced obesity, implantation of light-treated ASCs improved glucose tolerance and ameliorated systemic insulin resistance. Intriguingly, compared with non-light-treated ASCs, light-treated ASCs reduced monocyte infiltration and the levels of ATMs in EAT. Moreover, implantation of light-treated ASCs exerts more anti-inflammatory effects by suppressing M1 polarization and enhancing macrophage M2 polarization in EAT. Mass spectrometry revealed that light-treated human obese ASCs conditioned medium retained a more complete secretome with significant downregulation of pro-inflammatory cytokines and chemokines.
These data suggest that the combined application of photoactivation and ASCs using transplantation into dysfunctional adipose tissue contribute to selective suppression of inflammatory responses and protection from insulin resistance in obesity and type 2 diabetes.
© 2021. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
-
Stem Cells and Developmental Biology
In Journal of Veterinary Science on 1 May 2021 by An, J. H., Song, W. J., et al.
Malignant lymphoma is the most common hematopoietic malignancy in dogs, and relapse is frequently seen despite aggressive initial treatment. In order for the treatment of these recurrent lymphomas in dogs to be effective, it is important to choose a personalized and sensitive anticancer agent. To provide a reliable tool for drug development and for personalized cancer therapy, it is critical to maintain key characteristics of the original tumor.
In this study, we established a model of hybrid tumor/stromal spheroids and investigated the association between canine lymphoma cell line (GL-1) and canine lymph node (LN)-derived stromal cells (SCs).
A hybrid spheroid model consisting of GL-1 cells and LN-derived SC was created using ultra low attachment plate. The relationship between SCs and tumor cells (TCs) was investigated using a coculture system.
TCs cocultured with SCs were found to have significantly upregulated multidrug resistance genes, such as P-qp, MRP1, and BCRP, compared with TC monocultures. Additionally, it was revealed that coculture with SCs reduced doxorubicin-induced apoptosis and G2/M cell cycle arrest of GL-1 cells.
SCs upregulated multidrug resistance genes in TCs and influenced apoptosis and the cell cycle of TCs in the presence of anticancer drugs. This study revealed that understanding the interaction between the tumor microenvironment and TCs is essential in designing experimental approaches to personalized medicine and to predict the effect of drugs.
© 2021 The Korean Society of Veterinary Science.
-
FC/FACS
-
Cancer Research
-
Veterinary Research
In Sci Rep on 7 March 2022 by Nasiri, N., Hosseini, S., et al.
Fig.5.A

-
IHC
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 3
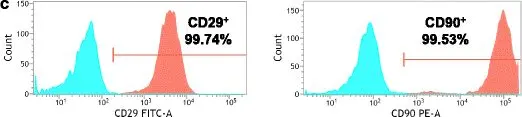
In Stem Cell Res Ther on 21 November 2017 by Yang, H., Wu, S., et al.
Fig.1.C

-
FC/FACS
-
Rattus norvegicus (Rat)
Collected and cropped from Stem Cell Res Ther by CiteAb, provided under a CC-BY license
Image 1 of 3
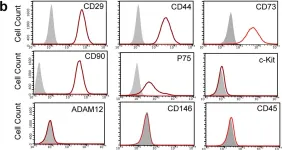
In Sci Rep on 10 January 2017 by Wang, D., Wang, A., et al.
Fig.3.B

-
FC/FACS
-
Rattus norvegicus (Rat)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 3