Acute lung injury (ALI), acute respiratory distress syndrome (ARDS) and pulmonary fibrosis remain major causes of morbidity, mortality and a healthcare burden in critically ill patient. There is an urgent need to identify factors causing susceptibility and for the design of new therapeutic agents. Here, we evaluate the effectiveness of the immunomodulatory neuropeptide cortistatin to regulate pulmonary inflammation and fibrosis in vivo.
ALI/ARDS and pulmonary fibrosis were induced experimentally in wild-type and cortistatin-deficient mice by pulmonary infusion of the bacterial endotoxin LPS or the chemotherapeutic drug bleomycin, and the histopathological signs, pulmonary leukocyte infiltration and cytokines, and fibrotic markers were evaluated.
Partially deficient mice in cortistatin showed exacerbated pulmonary damage, pulmonary inflammation, alveolar oedema and fibrosis, and subsequent increased respiratory failure and mortality when challenged to LPS or bleomycin, even at low doses. Treatment with cortistatin reversed these aggravated phenotypes and protected from progression to severe ARDS and fibrosis, after high exposure to both injury agents. Moreover, cortistatin-deficient pulmonary macrophages and fibroblasts showed exaggerated ex vivo inflammatory and fibrotic responses, and treatment with cortistatin impaired their activation. Finally, the protective effects of cortistatin in ALI and pulmonary fibrosis were partially inhibited by specific antagonists for somatostatin and ghrelin receptors.
We identified cortistatin as an endogenous inhibitor of pulmonary inflammation and fibrosis. Deficiency in cortistatin could be a marker of poor prognosis in inflammatory/fibrotic pulmonary disorders. Cortistatin-based therapies could emerge as attractive candidates to treat severe ALI/ARDS, including SARS-CoV-2-associated ARDS.
© 2021 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Product Citations: 13
Protective role of cortistatin in pulmonary inflammation and fibrosis.
In British Journal of Pharmacology on 1 November 2021 by Barriga, M., Benitez, R., et al.
-
Cardiovascular biology
-
Immunology and Microbiology
-
Pharmacology
In Cell Reports on 30 June 2020 by Yan, J., Pandey, S. P., et al.
IRF5 polymorphisms are associated with multiple immune-mediated diseases, including ulcerative colitis. IRF5 contributions are attributed to its role in myeloid lineages. How T cell-intrinsic IRF5 contributes to inflammatory outcomes is not well understood. We identify a previously undefined key role for T cell-intrinsic IRF5. In mice, IRF5 in CD4+ T cells promotes Th1- and Th17-associated cytokines and decreases Th2-associated cytokines. IRF5 is required for the optimal assembly of the TCR-initiated signaling complex and downstream signaling at early times, and at later times binds to promoters of Th1- and Th17-associated transcription factors and cytokines. IRF5 also regulates chemokine receptor-initiated signaling and, in turn, T cell migration. In vivo, IRF5 in CD4+ T cells enhances the severity of experimental colitis. Importantly, human CD4+ T cells from high IRF5-expressing disease-risk genetic carriers demonstrate increased chemokine-induced migration and Th1/Th17 cytokines and reduced Th2-associated and anti-inflammatory cytokines. These data demonstrate key roles for T cell-intrinsic IRF5 in inflammatory outcomes.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
-
ELISA
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Anticancer and Apoptogenic Effect of Graviola and Low-Dose Radiation in Tumor Xenograft in Mice.
In Integrative Cancer Therapies on 5 June 2020 by El Tawiil, G. A., Noaman, E. A., et al.
Background:Annona muricata (graviola) has been claimed for its potential against various diseases including cancer. Objective: The present study aimed to investigate the anticancer effect of graviola extract on Ehrlich solid tumor (EST) mice along with or without a low dose of γ radiation (LDR). Methods: Mice were treated with graviola 50 mg/kg body weight orally for 30 days after EST induction and exposed to γ-ray (2 Gy/week for 3 weeks). Cell cycle, CD44, TGF-β, Bcl-2, and annexin V were determined in tumor tissue. Results: The result obtained showed a significant decrease (P < .05) of tumor size in 28 graviola-treated EST-bearing mice group (EG) or graviola-treated and irradiated EST-30-bearing mice (EGR) groups versus the EST group. The large number of cells in the sub-G0/G1 population and low cell number at S and M phases signify tumor cell apoptosis and inhibition of cell division in EG or EGR groups. Additionally, significant increases in the expression of CD44 and TGF-β were recorded in EST mice as compared with EG or EGR mice. Furthermore, EST mice exhibited a decrease in the apoptotic marker annexin v and increase in antiapoptotic Bcl-2 compared with EG and EGR mice. Conclusion: It could be suggested that graviola exerts its antitumor effect throughout the regulation of the tumor cell cycle as well as inducing apoptotic signals. The combined treatment of graviola and LDR augments their effect on tumor proliferation.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
In Scientific Reports on 9 April 2020 by Nelson, A. S., Maddaloni, M., et al.
Antigen (Ag)-specific tolerization prevents type 1 diabetes (T1D) in non-obese diabetic (NOD) mice but proved less effective in humans. Several auto-Ags are fundamental to disease development, suggesting T1D etiology is heterogeneous and may limit the effectiveness of Ag-specific therapies to distinct disease endotypes. Colonization factor antigen I (CFA/I) fimbriae from Escherichia coli can inhibit autoimmune diseases in murine models by inducing bystander tolerance. To test if Ag-independent stimulation of regulatory T cells (Tregs) can prevent T1D onset, groups of NOD mice were orally treated with Lactococcus lactis (LL) expressing CFA/I. LL-CFA/I treatment beginning at 6 weeks of age reduced disease incidence by 50% (p < 0.05) and increased splenic Tregs producing both IL-10 and IFN-γ 8-fold (p < 0.005) compared to LL-vehicle treated controls. To further describe the role of these Tregs in preventing T1D, protective phenotypes were examined at different time-points. LL-CFA/I treatment suppressed splenic TNF-α+CD8+ T cells 6-fold at 11 weeks (p < 0.005) and promoted a distinct microbiome. At 17 weeks, IFN-γ+CD4+ T cells were suppressed 10-fold (p < 0.005), and at 30 weeks, pancreatic Tbet+CD4+ T cells were suppressed (p < 0.05). These results show oral delivery of modified commensal organisms, such as LL-CFA/I, may be harnessed to restrict Th1 cell-mediated immunity and protect against T1D.
-
Immunology and Microbiology
In Frontiers in Cellular and Infection Microbiology on 24 January 2020 by Berdasco, C., Duhalde Vega, M., et al.
Shiga toxin (Stx) produced by enterohemorrhagic E. coli produces hemolytic uremic syndrome and encephalopathies in patients, which can lead to either reversible or permanent neurological abnormalities, or even fatal cases depending on the degree of intoxication. It has been observed that the inflammatory component plays a decisive role in the severity of the disease. Therefore, the objective of this work was to evaluate the behavior of microglial cell primary cultures upon Stx2 exposure and heat shock or lipopolysaccharide challenges, as cues which modulate cellular environments, mimicking fever and inflammation states, respectively. In these contexts, activated microglial cells incorporated Stx2, increased their metabolism, phagocytic capacity, and pro-inflammatory profile. Stx2 uptake was associated to receptor globotriaosylceramide (Gb3)-pathway. Gb3 had three clearly distinguishable distribution patterns which varied according to different contexts. In addition, toxin uptake exhibited both a Gb3-dependent and a Gb3-independent binding depending on those contexts. Altogether, these results suggest a fundamental role for microglial cells in pro-inflammatory processes in encephalopathies due to Stx2 intoxication and highlight the impact of environmental cues.
Copyright © 2020 Berdasco, Duhalde Vega, Rosato-Siri and Goldstein.
-
Immunology and Microbiology
-
Neuroscience
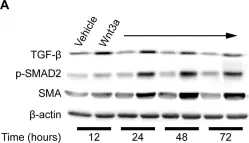
In PLoS One on 26 May 2011 by Carthy, J. M., Garmaroudi, F. S., et al.
Fig.6.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 1