Brain arteriovenous malformations (bAVMs) are anomalies forming vascular tangles connecting the arteries and veins, which cause hemorrhagic stroke in young adults. Current surgical approaches are highly invasive, and alternative therapeutic methods are warranted. Recent genetic studies identified KRAS mutations in endothelial cells of bAVMs; however, the underlying process leading to malformation in the postnatal stage remains unknown. Here we established a mouse model of bAVM developing during the early postnatal stage. Among 4 methods tested, mutant KRAS specifically introduced in brain endothelial cells by brain endothelial cell-directed adeno-associated virus (AAV) and endothelial cell-specific Cdh5-CreERT2 mice successfully induced bAVMs in the postnatal period. Mutant KRAS led to the development of multiple vascular tangles and hemorrhage in the brain with increased MAPK/ERK signaling and growth in endothelial cells. Three-dimensional analyses in cleared tissue revealed dilated vascular networks connecting arteries and veins, similar to human bAVMs. Single-cell RNA-Seq revealed dysregulated gene expressions in endothelial cells and multiple cell types involved in the pathological process. Finally, we employed CRISPR/CasRx to knock down mutant KRAS expression, which efficiently suppressed bAVM development. The present model reveals pathological processes that lead to postnatal bAVMs and demonstrates the efficacy of therapeutic strategies with CRISPR/CasRx.
Product Citations: 145
In JCI Insight on 22 November 2024 by Saito, S., Nakamura, Y., et al.
-
IHC
-
Mus musculus (House mouse)
In Journal of Neuroimmune Pharmacology : the Official Journal of the Society On NeuroImmune Pharmacology on 20 August 2024 by Ruiz-Cantero, M. C., Entrena, J. M., et al.
The mechanisms for neuropathic pain amelioration by sigma-1 receptor inhibition are not fully understood. We studied genome-wide transcriptomic changes (RNAseq) in the dorsal root ganglia (DRG) from wild-type and sigma-1 receptor knockout mice prior to and following Spared Nerve Injury (SNI). In wildtype mice, most of the transcriptomic changes following SNI are related to the immune function or neurotransmission. Immune function transcripts contain cytokines and markers for immune cells, including macrophages/monocytes and CD4 + T cells. Many of these immune transcripts were attenuated by sigma-1 knockout in response to SNI. Consistent with this we found, using flow cytometry, that sigma-1 knockout mice showed a reduction in macrophage/monocyte recruitment as well as an absence of CD4 + T cell recruitment in the DRG after nerve injury. Sigma-1 knockout mice showed a reduction of neuropathic (mechanical and cold) allodynia and spontaneous pain-like responses (licking of the injured paw) which accompany the decreased peripheral neuroinflammatory response after nerve injury. Treatment with maraviroc (a CCR5 antagonist which preferentially inhibits CD4 + T cells in the periphery) of neuropathic wild-type mice only partially replicated the sigma-1 knockout phenotype, as it did not alter cold allodynia but attenuated spontaneous pain-like responses and mechanical hypersensitivity. Therefore, modulation of peripheral CD4 + T cell activity might contribute to the amelioration of spontaneous pain and neuropathic tactile allodynia seen in the sigma-1 receptor knockout mice, but not to the effect on cold allodynia. We conclude that sigma-1 receptor inhibition decreases DRG neuroinflammation which might partially explain its anti-neuropathic effect.
© 2024. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
-
IHC
-
Neuroscience
In The Journal of Neuroscience on 10 July 2024 by Weinberg, R. L., Kim, S., et al.
SLURP1 and SLURP2 are both small secreted members of the Ly6/u-PAR family of proteins and are highly expressed in keratinocytes. Loss-of-function mutations in SLURP1 lead to a rare autosomal recessive palmoplantar keratoderma (PPK), Mal de Meleda (MdM), which is characterized by diffuse, yellowish palmoplantar hyperkeratosis. Some individuals with MdM experience pain in conjunction with the hyperkeratosis that has been attributed to fissures or microbial superinfection within the affected skin. By comparison, other hereditary PPKs such as pachyonychia congenita and Olmsted syndrome show prevalent pain in PPK lesions. Two mouse models of MdM, Slurp1 knock-out and Slurp2X knock-out, exhibit robust PPK in all four paws. However, whether the sensory experience of these animals includes augmented pain sensitivity remains unexplored. In this study, we demonstrate that both models exhibit hypersensitivity to mechanical and thermal stimuli as well as spontaneous pain behaviors in males and females. Anatomical analysis revealed slightly reduced glabrous skin epidermal innervation and substantial alterations in palmoplantar skin immune composition in Slurp2X knock-out mice. Primary sensory neurons innervating hindpaw glabrous skin from Slurp2X knock-out mice exhibit increased incidence of spontaneous activity and mechanical hypersensitivity both in vitro and in vivo. Thus, Slurp knock-out mice exhibit polymodal PPK-associated pain that is associated with both immune alterations and neuronal hyperexcitability and might therefore be useful for the identification of therapeutic targets to treat PPK-associated pain.
Copyright © 2024 the authors.
-
Mus musculus (House mouse)
-
Neuroscience
Lysophosphatidic acid receptor 1 influences disease severity in a mouse model of multiple sclerosis
Preprint on BioRxiv : the Preprint Server for Biology on 2 June 2024 by Uemura, N., Matsuo, N., et al.
Multiple sclerosis (MS), a chronic inflammatory disease affecting the central nervous system (CNS), is characterized by demyelination and axonal degeneration. Current treatments, which focus mainly on reducing lymphocyte infiltration into the CNS, are insufficient due to serious side effects and limited effectiveness; thus, identifying drugs with new mechanisms of action is crucial. Lysophosphatidic acid (LPA), a bioactive lipid produced by the enzyme autotaxin, may play a role in MS pathogenesis. Specifically, the LPA 1 subtype of LPA receptors is linked to release of inflammatory cytokines in the CNS, and to demyelination in the peripheral nervous system. Our study investigated the role of LPA 1 in a mouse model of MS. Knocking out the LPA 1 gene in mice with experimental autoimmune encephalomyelitis improved clinical outcomes and reduced demyelination. Additionally, the absence of LPA 1 reduced activation of Iba1-positive cells. Treatment with AM095, an LPA 1 antagonist, tended to improve clinical outcomes and reduce levels of inflammatory mediators. These findings indicate that activation of LPA 1 contributes to MS pathogenesis by promoting microglial activation and infiltration of peripheral immune cells.
-
IHC
-
Mus musculus (House mouse)
Preprint on BioRxiv : the Preprint Server for Biology on 1 June 2024 by Ohashi, K., Uemura, N., et al.
Oligodendrocyte precursor cells (OPCs) are a type of glial cell that differentiates into mature oligodendrocytes, a cell type that contributes to myelination, but their roles in the pathologies are not fully understood. Activities other than differentiation into oligodendrocytes have recently been reported for OPCs present in the inflammatory milieu, but intervention studies using animal models are lacking. This study aimed to explore the role of OPCs in mouse MS model experimental autoimmune encephalomyelitis (EAE). Using inducible diphtheria toxin receptor-expressing transgenic mice, platelet-derived growth factor receptor A (PDGFRα) + OPCs were depleted in EAE mice. Surprisingly, OPC depletion in the acute phase improved clinical scores and reduced demyelination. Major histocompatibility complex (MHC) class II was reduced in the spinal cord, whereas astrocyte marker and blood–spinal cord barrier tight junction and adhesion molecule expressions were unaffected after OPC depletion. The numbers of T cells and IL17-expressing Th17 cells were decreased in the spinal cords of the OPC-depleted group. MHC class II expression in spinal cord macrophages was consistently decreased by OPC depletion. These data suggest that in the acute phase of EAE, OPCs are involved in activation of infiltrated macrophages and induce subsequent T cell activation and neuroinflammation. Although the precise mechanisms remain unclear, this implies that OPCs exist not only as the source for oligodendrocytes but also play a pivotal role in central nervous system (CNS) autoimmune inflammation. Table of Contents Image MAIN POINTS OPC depletion in the acute phase of EAE improved clinical scores and reduced demyelination. OPC depletion in the spinal cord suppressed Antigen presentation via MHC class II. OPCs are involved in activation of infiltrated macrophages and induce subsequent T cell activation and neuroinflammation.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
-
Neuroscience
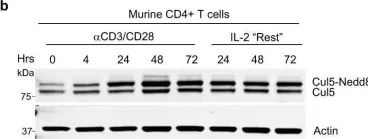
In Nat Commun on 19 May 2022 by Kumar, B., Field, N. S., et al.
Fig.1.B

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 4
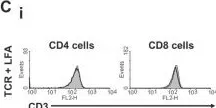
In PLoS One on 31 July 2013 by Schroder, P. M., Khattar, M., et al.
Fig.6.C

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4
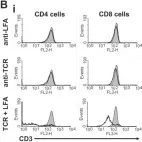
In PLoS One on 31 July 2013 by Schroder, P. M., Khattar, M., et al.
Fig.6.A

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4
In PLoS One on 31 July 2013 by Schroder, P. M., Khattar, M., et al.
Fig.6.B

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4