Homozygous knockout of scavenger receptor class B type I (SR-B1) in mice with atherogenic mutations (such as knockout of the apolipoprotein E or low density lipoprotein receptor genes) results in spontaneous or diet-induced coronary heart disease characterized by atherosclerosis development in the aortic sinus and coronary arteries, platelet accumulation in coronary artery plaques, myocardial fibrosis, and early death. However, the extent of coronary artery atherothrombosis and myocardial fibrosis in mice lacking SR-B1 alone (homozygous SR-B1 knockout mice) has not been examined. Although age is a major risk factor for coronary artery disease, few studies directly examine the effects of age on susceptibility to atherosclerosis or coronary artery atherothrombosis and myocardial fibrosis in mice. Therefore, we set out to examine the effects of age on diet-induced atherosclerosis in female homozygous SR-B1 knockout mice.
SR-B1 knockout mice exhibited little-to-no aortic sinus or coronary artery atherosclerosis at 52 weeks of age, when fed a normal diet. However when fed a high-fat, high-cholesterol, cholate-containing (HFCC) diet for 12 weeks from either 14 weeks of age (26-week-old at analysis) or 40 weeks of age (52-week-old at analysis), they developed similar degrees of atherosclerosis in their aortic sinuses. Interestingly, the older aged SR-B1 knockout mice exhibited increased coronary artery atherosclerosis, increased vascular cell adhesion molecule 1 levels and platelet accumulation in coronary arteries, and increased myocardial fibrosis and plasma levels of cardiac troponin I compared to the younger aged mice. Older-aged HFCC diet-fed SR-B1 knockout mice also exhibited reduced survival to humane endpoint. Moreover, older-aged HFCC diet-fed SR-B1 knockout mice exhibited a greater inflammatory state with increased levels of circulating interleukin-6, tumour necrosis factor alpha, and neutrophils, despite plasma lipid levels being unchanged. Consistent with the increased circulating neutrophils, older-aged HFCC diet-fed SR-B1 knockout mice exhibited increased accumulation of the neutrophil marker myeloperoxidase and increased neutrophil extracellular traps in atherosclerotic plaques in the aortic sinus and increased abundance of atherosclerotic coronary arteries containing neutrophil extracellular traps.
HFCC diet-fed homozygous SR-B1 knockout mice develop occlusive coronary artery atherothrombosis and myocardial fibrosis in an age-dependent manner, and exhibit an increased inflammatory state with older age. Therefore, aged SR-B1 knockout mice may prove to be an attractive mouse model to analyze age-dependent mechanisms associated with coronary artery disease development, which may facilitate the discovery of more effective therapeutics to treat cardiovascular disease.
Copyright: © 2025 Lee et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Product Citations: 128
In PLoS ONE on 22 May 2025 by Lee, S. K., Xiong, T., et al.
-
Cardiovascular biology
Preprint on BioRxiv : the Preprint Server for Biology on 10 March 2025 by Chen, K., Li, X., et al.
Lipid nanoparticle (LNP)-based mRNA therapeutics, highlighted by the success of SARS-CoV-2 vaccines, face challenges due to inflammation caused by ionizable lipids. These ionizable lipids can activate the immune system, particularly when co-delivered with nucleic acids, leading to undesirable inflammatory responses. We introduce a novel class of anti-inflammatory ionizable lipids functionalized with hydroxychloroquine (HCQ), which suppresses both lipid-induced and nucleic acid-induced immune activation. These HCQ-functionalized LNPs (HL LNPs) exhibit reduced proinflammatory responses while maintaining efficient mRNA delivery. Structural and physicochemical analyses revealed that HCQ-functionalization results in a distinct particle structure with significantly improved stability. The efficacy of HL LNPs was demonstrated across various therapeutic contexts, including a prophylactic vaccination model against varicella-zoster virus (VZV) and CRISPR-Cas9 gene editing targeting PCSK9. Notably, HL LNPs showed robust mRNA expression after repeated administration, addressing concerns of inflammation and ensuring sustained therapeutic effects. These findings highlight the potential of HCQ-functionalized LNPs in expanding the safe use of mRNA therapeutics, particularly for applications requiring repeated dosing and in scenarios where inflammation-induced side effects must be minimized.
-
Genetics
-
Immunology and Microbiology
In Journal of Inflammation Research on 24 February 2025 by Jang, J. H., Song, Y., et al.
Persistent inflammation resulting from injury, infection, or arthritis contributes to both peripheral and central sensitization. Various combinations of natural extracts have been explored to minimize the side effects associated with conventional medications. Shinbaro, which has traditionally been used in Eastern medicine to treat inflammatory conditions, was chosen due to its known anti-inflammatory properties. However, previous studies have not yet investigated the combined administration of celecoxib and Shinbaro for their anti-inflammatory and analgesic effects. In this study, we examined the anti-inflammatory and analgesic effects of combining celecoxib with Shinbaro in a complete Freund's adjuvant (CFA)-induced inflammatory pain model.
We randomly assigned 66 mice to 6 groups (n = 11 per group) and administered intraplantar injections of 100 μL CFA or saline into their right hind paw, followed by oral administration of Shinbaro (100 mg/kg), celecoxib (15 or 30 mg/kg), or both 30 minutes later. Behavioral assessments were conducted blindly at baseline and on days 1, 3, and 7 post-injection. The right hind paw and spinal cord were harvested 3 days post-injection to examine the molecular mechanisms, including macrophage infiltration in the right hind paw, as well as glial cell activation and inflammatory cytokine levels in the spinal cord. Statistical analysis was performed using Tukey's post-hoc test.
The combination of Shinbaro (100 mg/kg) and celecoxib (15 mg/kg) synergistically reduced mechanical hyperalgesia and paw edema by preventing the conversion of monocytes to macrophages and inhibiting macrophage infiltration. Moreover, it decreased the expression of pro-inflammatory cytokines and mediators in the spinal cord by inhibiting spinal microglial activation.
The combination of Shinbaro and celecoxib demonstrates significant anti-inflammatory and analgesic effects, suggesting its potential for managing inflammatory pain with fewer side effects than conventional therapies.
© 2025 Jang et al.
-
Immunology and Microbiology
In Cell Reports on 24 December 2024 by Ma, R., Prigge, A. D., et al.
Forkhead box P3 (Foxp3)+ regulatory T cells (Tregs) resolve acute inflammation and repair the injured lung after viral pneumonia. Vimentin is a critical protein in the distal pole complex (DPC) of Tregs. This study reveals the inhibitory effect of vimentin on the suppressive and reparative capacity of Tregs. Treg-specific deletion of vimentin increases Helios+interleukin-18 receptor (IL-18R)+ Tregs, suppresses inflammatory immune cells, and enhances tissue repair, protecting Vimfl/flFoxp3YFP-cre mice from influenza-induced lung injury and mortality. Mechanistically, vimentin suppresses the induction of amphiregulin, an epidermal growth factor receptor (EGFR) ligand necessary for tissue repair, by sequestering IL-18R to the DPC and restricting receptor-ligand interactions. We propose that vimentin in the DPC of Tregs functions as a molecular switch, which could be targeted to regulate the immune response and enhance tissue repair in patients with severe viral pneumonia.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Immunology and Microbiology
In Cancer Gene Therapy on 1 November 2024 by Ferrantelli, F., Manfredi, F., et al.
We previously developed an innovative strategy to induce CD8+ T lymphocyte-immunity through in vivo engineering of extracellular vesicles (EVs). This approach relies on intramuscular injection of DNA expressing antigens of interest fused at a biologically-inactive HIV-1 Nef protein mutant (Nefmut). Nefmut is very efficiently incorporated into EVs, thus conveying large amounts of fusion proteins into EVs released by transfected cells. This platform proved successful against highly immunogenic tumor-specific antigens. Here, we tested whether antigen-specific CD8+ T cell immune responses induced by engineered EVs can counteract the growth of tumors expressing two "self" tumor-associated antigens (TAAs): HOXB7 and Her2/neu. FVB/N mice were injected with DNA vectors expressing Nefmut fused to HOXB7 or Her2/neu, singly and in combination, before subcutaneous implantation of breast carcinoma cells co-expressing HOXB7 and Her2/neu. All mice immunized with the combination vaccine remained tumor-free, whereas groups vaccinated with single Nefmut-fused antigens were only partly protected, with stronger antitumor effects in Her2/neu-immunized mice. Double-vaccinated mice also controlled tumor growth upon a later tumor cell re-challenge. Importantly, co-vaccination also contained tumors in a therapeutic immunization setting. These results showed the efficacy of EV-based vaccination against two TAAs, and represent the first demonstration that HOXB7 may be targeted in multi-antigen immunotherapy strategies.
© 2024. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
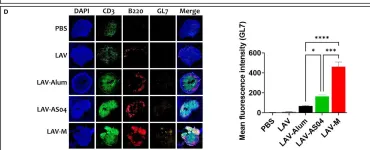
In Front Immunol on 28 October 2022 by Varma, V. P., Kadivella, M., et al.
Fig.4.D

-
ICC-IF
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 1