Coronary arteries develop under constant mechanical stress. However, the role of mechanosensitive ion channels in this process remains poorly understood. Here we show that the ion channel PIEZO2, which responds to mechanical stimuli, is expressed in specific coronary endothelial cell populations during a critical phase of coronary vasculature remodeling. These Piezo2+ coronary endothelial cells show distinct transcriptional profiles and have mechanically activated ionic currents. Strikingly, PIEZO2 loss-of-function mouse embryos and mice with human pathogenic variants of PIEZO2 show abnormal coronary vessel development and cardiac left ventricular hyperplasia. We conclude that an optimal balance of PIEZO2 channel function contributes to proper coronary vessel formation, structural integrity and remodeling, and is likely to support normal cardiac function. Our study highlights the importance of mechanical cues in cardiovascular development and suggests that defects in this mechanosensing pathway may contribute to congenital heart conditions.
© 2025. The Author(s).
Product Citations: 179
Mechanosensitive PIEZO2 channels shape coronary artery development.
In Nat Cardiovasc Res on 27 June 2025 by Pampols-Perez, M., Fürst, C., et al.
-
Cardiovascular biology
In GeroScience on 1 June 2025 by Najari Beidokhti, M., Villalba, N., et al.
Cellular senescence contributes to inflammation and organ dysfunction during aging. While this process is generally characterized by irreversible cell cycle arrest, its morphological features and functional impacts vary in different cells from various organs. In this study, we examined the expression of multiple senescent markers in the lungs of young and aged humans and mice, as well as in mouse lung endothelial cells cultured with a senescence inducer, suberoylanilide hydroxamic acid (SAHA), or doxorubicin (DOXO). We detected increased levels of p21, γH2AX, and SA-β-Gal and decreased Ki-67 and Lamin B1 in aged lungs and senescent lung endothelial cells. Importantly, the expression of senescent markers was associated with an inflammatory response in aged mouse lungs characterized by neutrophil infiltration, increased expression of intercellular adhesion molecule 1 (ICAM-1), and decreased protein levels of VE-cadherin and ZO-1. As the latter two are critical constituents of endothelial cell-cell junctions, we hypothesized that their decreased expression could lead to compromised junction barrier integrity. Indeed, senescent endothelial cells (ECs) exhibited impaired barrier properties, as measured by increased permeability to solutes of small size (3-kD) and albumin (70-kD). When co-cultured with neutrophils, senescent ECs and their supernatant promoted neutrophil chemotaxis and trans-endothelial migration. Taken together, our results suggest that lung EC senescence weakens cell-cell junctions, impairs barrier function, and promotes neutrophil adhesion and migration, which may contribute to the development of inflammation and related pathologies in the lungs during aging.
© 2025. The Author(s).
In Scientific Data on 19 May 2025 by Kumar Maji, R., Fischer, A., et al.
Acute myocardial infarction (AMI) is a leading cause of mortality worldwide. MicroRNAs (miRNAs), among other small non-coding RNAs, shape the transcriptome and control cellular functions. Although single-cell technologies are now available to study myocardial ischemia response, the study of small RNA regulation is limited by depth of expression, capture efficiency and lack of full coverage of transcripts. In addition, the kinetic expression of miRNAs is unknown. Using paired small and total RNA sequencing, we built an expression atlas to study the temporal dynamics of miRNAs and genes in four major heart cell types after AMI. Expression dynamics reveal enriched functions highlighting cell type-specific AMI stress responses. Many deregulated mouse genes after AMI overlap with known human cardiovascular disease genes. The dataset is highly valuable for additional research on small and long non-coding RNAs, such as regulation of RNA variants by splicing or alternative ORFs. All in all, the RNA expression atlas provides a useful resource to study different roles of RNAs in major cell types of the heart after AMI.
© 2025. The Author(s).
-
Mus musculus (House mouse)
-
Cardiovascular biology
-
Genetics
In IScience on 17 January 2025 by Kiesworo, K., Agius, T., et al.
Aging is accompanied by a decline in neovascularization potential and increased susceptibility to ischemic injury. Here, we confirm the age-related impaired neovascularization following ischemic leg injury and impaired angiogenesis. The age-related deficits in angiogenesis arose primarily from diminished EC proliferation capacity, but not migration or VEGF sensitivity. Aged EC harvested from the mouse skeletal muscle displayed a pro-angiogenic gene expression phenotype, along with considerable changes in metabolic genes. Metabolomics analysis and 13C glucose tracing revealed impaired ATP production and blockade in glycolysis and TCA cycle in late passage HUVECs, which occurred at nicotinamide adenine dinucleotide (NAD⁺)-dependent steps, along with NAD+ depletion. Supplementation with nicotinamide mononucleotide (NMN), a precursor of NAD⁺, enhances late-passage EC proliferation and sprouting angiogenesis from aged mice aortas. Taken together, our study illustrates the importance of NAD+-dependent metabolism in the maintenance of EC proliferation capacity with age, and the therapeutic potential of NAD precursors.
© 2024 The Author(s).
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
IFT20 regulates lymphatic endothelial cell-cell junctions via endocytic trafficking of VE-cadherin
Preprint on BioRxiv : the Preprint Server for Biology on 15 January 2025 by Paulson, D., Majumder, A., et al.
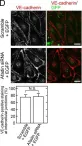
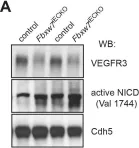
Intraflagellar transport (IFT) proteins are required for the assembly and function of primary cilia. They also regulate non-ciliary polarized vesicular traffic, such as T cell receptor recycling. We recently reported that lymphatic endothelial cells assemble primary cilia and express IFT proteins. Here, we report that IFT20 regulates vascular endothelial cadherin (VE-cadherin) localization at adherens junctions. IFT20 deletion caused discontinuous, button-like interendothelial junctions. This resulted in excessive lymphangiogenesis and impaired lymph drainage in mice. In vitro, VEGF-C treatment of IFT20 KD primary human dermal lymphatic endothelial cells caused accumulation of VE-cadherin in RAB5+ endosomes and enhanced and sustained VEGFR-3 signaling. Our findings are consistent with a model in which IFT20 promotes recycling of VE-cadherin to the adherens junction where it sequesters VEGFR-3 at the cell surface, thereby limiting pro-lymphangiogenic signaling. In the absence of IFT20, intercellular junctions are destabilized, pro-lymphangiogenic VEGFR-3 signaling is enhanced, and lymph transport is impaired by intracellular sequestration of VE-cadherin. This study elucidates the function of an IFT protein in lymphatic endothelial cells and provides mechanistic insight into the processes that regulate lymphatic endothelial cell-cell junctions and lymphangiogenic signaling.
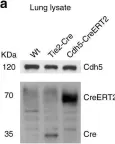
In Nat Commun on 22 May 2019 by Fernández-Chacón, M., Casquero-Garcia, V., et al.
Fig.7.A

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 6
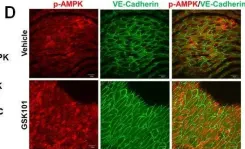
In Oncotarget on 21 June 2016 by Xu, S., Liu, B., et al.
Fig.4.D

-
IHC-IF
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 6
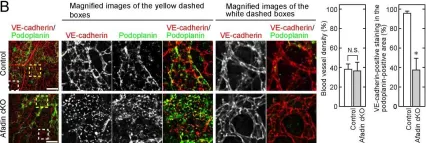
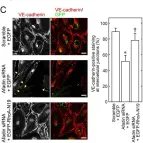
In PLoS One on 11 July 2013 by Majima, T., Takeuchi, K., et al.
Fig.2.B

-
IHC-IF
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 6
In PLoS One on 11 July 2013 by Majima, T., Takeuchi, K., et al.
Fig.5.C

-
ICC-IF
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 6
In PLoS One on 11 July 2013 by Majima, T., Takeuchi, K., et al.
Fig.5.D

-
ICC-IF
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 6
In PLoS One on 1 August 2012 by Izumi, N., Helker, C., et al.
Fig.4.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 6