Parkinson's disease (PD) patients frequently exhibit vitamin D deficiency and an imbalance in T helper 17 (Th17) and regulatory T (Treg) cells, which may contribute to disease pathogenesis. Preclinical evidence suggests vitamin D regulates Th17/Treg balance, but the therapeutic effects of supplementation in PD remain unestablished. This randomized controlled trial investigated peripheral blood levels of vitamin D, Treg, and Th17 cells in PD patients, examined their associations with clinical outcomes, and assessed vitamin D3 supplementation's effects on immunological and motor functions. In this randomized, double-blind, placebo-controlled trial, 51 PD patients and 50 healthy controls (HCs) were enrolled. Thirty PD patients with vitamin D deficiency were randomized to receive vitamin D3 (n = 15) or placebo (vegetable oil, n = 15) for three months. Serum 25(OH)D3 levels were measured by electrochemiluminescence, and Th17/Treg cells were analyzed by flow cytometry. Motor and non-motor symptoms were assessed using standardized scales. Vitamin D3 supplementation significantly increased 25(OH)D3 levels (p < 0.05), reduced Th17 cells (4.62 ± 1.09 to 3.25 ± 1.14, p = 0.003), and elevated Tregs (3.25 ± 0.90 to 4.52 ± 0.95, p = 0.003). Motor function (UPDRS and UPDRS-III) improved in the vitamin D3 group (p < 0.001), while no changes were observed in the placebo group. This preliminary study suggests that vitamin D3 supplementation may restore Th17/Treg balance and potentially alleviate motor symptoms in vitamin D-deficient PD patients, indicating a possible therapeutic strategy.Trial registration: ClinicalTrials.gov: NCT:06539260. Registered 05 August 2024 - Retrospectively registered, https://clinicaltrials.gov/study/NCT06539260 .
© 2025. The Author(s).
Product Citations: 168
In Scientific Reports on 11 July 2025 by Li, D., Ma, X., et al.
-
Immunology and Microbiology
-
Neuroscience
In World Journal of Surgical Oncology on 1 July 2025 by Yin, Z., Xiao, Y., et al.
-
Cancer Research
In IScience on 16 May 2025 by Lin, S., Ma, Y., et al.
This phase 1b/2 clinical trial (NCT04775680) evaluated the safety, efficacy, pharmacokinetics and pharmacodynamics of ADG106, a ligand-blocking agonistic antibody targeting CD137 (4-1BB), combined with toripalimab in patients with advanced malignancies. ADG106 0.75-3 mg/kg plus toripalimab 240 mg were administered every 3 weeks. One dose-limiting toxicity occurred in 1 subject at 1.5 mg/kg and 2 in another subject at 3 mg/kg. Grade ≥ 3 treatment related adverse events occurred in 4/25 patients (16%). The overall disease control rate was 29.2% (7/24), including 1 partial response (PR) patient with a duration of response and a progression-free survival of 17.6 and 24.5 months. Circulating biomarkers suggested increased soluble CD137, CD3-CD16+CD56+ natural killer (NK) cells, interferon γ (IFN-γ), TNFα, and IL-6 after therapy. Elevated baseline memory T cells and PD-L1, activation of immune-related pathways, along with enhanced T cell proliferation and increased IFN-γ following treatment were observed in the PR patient. ADG106 in combination with toripalimab demonstrated a manageable safety profile but no efficacy conclusions could be drawn.
© 2025 Published by Elsevier Inc.
-
Cancer Research
γδ T Are Significantly Impacted by CLL Burden but Only Mildly Influenced by M-MDSCs.
In Cancers on 14 January 2025 by Zarobkiewicz, M., Kowalska, W., et al.
The current study explores the impact of CLL on γδ T cells and, in an attempt to better understand the sources of immunosuppression, assesses the impact of M-MDSCs on γδ T cells in vitro.
The study included 163 CLL patients and 34 healthy volunteers. γδ T cells were screened with flow cytometry, including NKG2D, Fas, FasL, and TRAIL staining. Additionally, to deepen understanding of the immunosuppressive impact of CLL on γδ T, a set of in vitro co-cultures of γδ T and M-MDSCs was performed.
RNAseq revealed significant, though relatively minor, changes in the transcriptome. Functional analyses showed a minor drop in cytotoxic potential against CLL cells. Finally, depletion of M-MDSCs from CLL-derived peripheral blood mononuclear cells did not restore γδ T cells' proliferative response.
Altogether, this suggests a minor impact of M-MDSCs on activated γδ T. Thus, it seems probable that other mechanisms than M-MDSCs mediate the negative impact of CLL on circulating γδ T cells.
-
Homo sapiens (Human)
-
Cancer Research
In Oncoimmunology on 31 December 2024 by Matsumoto, S., Tsujikawa, T., et al.
Neoantigen-reactive CD4+ T cells play a key role in the anti-tumor immune response. However, the majority of epithelial tumors are negative for HLA class II (HLA-II) surface expression, and less is known about the processing of HLA-II antigens. Here, we directly identified naturally presented HLA-II neoantigens in HLA-II negative colorectal cancer (CRC) tissue using a proteogenomic approach. The neoantigens were immunogenic and induced patient CD4+ T cells with a Th1-like memory phenotype that produced IFN-γ, IL2 and TNF-α. Multiplex immunohistochemistry (IHC) demonstrated an interaction between Th cells and HLA-II-positive antigen-presenting cells (APCs) at the invasive margin and within the tertiary lymphoid structures (TLS). In our CRC cohort, the density of stromal APCs was associated with HLA-II antigen presentation in the tumor microenvironment (TME), and the number of TLS was positively correlated with the number of somatic mutations in the tumors. These results demonstrate the presence of neoantigen-specific CD4+ surveillance in HLA-II-negative CRC and suggest a potential role for macrophages and dendritic cells (DCs) at the invasive margin and in TLS for antigen presentation. Stromal APCs in the TME can potentially be used as a source for HLA-II neoantigen identification.
-
Cancer Research
-
Immunology and Microbiology
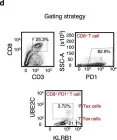
In Elife on 22 February 2023 by Cheng, D. N., Qiu, K., et al.
Fig.4.D

-
FC/FACS
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 5
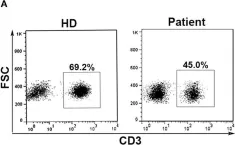
In Front Immunol on 28 October 2019 by Chen, P. Y., Wu, C. Y., et al.
Fig.1.A

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 5
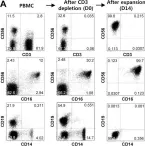
In Front Immunol on 28 October 2019 by Chen, P. Y., Wu, C. Y., et al.
Fig.1.C

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 5
In J Inflamm (Lond) on 2 April 2014 by Brittan, M., Barr, L. C., et al.
Fig.2.B

-
FC/FACS
-
Collected and cropped from J Inflamm (Lond) by CiteAb, provided under a CC-BY license
Image 1 of 5
In PLoS One on 18 January 2013 by Lim, O., Lee, Y., et al.
Fig.1.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 5