Aberrant vascular systems are significant indicators of cancer and play pivotal roles in tumor immunomodulation. However, the role of PD-L1 expressed on vascular endothelial cells (VECs) in the tumor immune microenvironment of nasopharyngeal carcinoma (NPC), as well as its correlation with patient prognosis, remains unclear. According to in vitro experiments conducted in our research, NPC tumor supernatants could upregulate PD-L1 expression on HUVECs, and the upregulated PD-L1 could bind to PD-1 on T cells leading to diminished T cell killing. The results of animal experiments similarly showed that elevated levels of PD-L1 on tumor VECs hindered the anti-tumor effectiveness of T cells, resulting in immune evasion and tumor progression. Furthermore, PD-L1 expression on tumor VECs served as a valuable prognostic marker, with heightened expression linked to poorer prognosis in NPC patients. Mechanistically, we discovered that the interaction between NF-κB and STAT3 signaling pathways may contribute significantly to the up-regulation of PD-L1 on VECs in NPC. Together, our work provides novel insights into identifying prognostic markers and strategies for reversing immune evasion mechanisms in NPC.
© 2025. The Author(s).
Product Citations: 94
In Cell Death & Disease on 25 February 2025 by Wang, Y., Chen, Y., et al.
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
In Journal of Translational Medicine on 27 September 2024 by Court, A. C., Parra-Crisóstomo, E., et al.
Apoptosis, a form of programmed cell death, is critical for the development and homeostasis of the immune system. Chimeric antigen receptor T (CAR-T) cell therapy, approved for hematologic cancers, retains several limitations and challenges associated with ex vivo manipulation, including CAR T-cell susceptibility to apoptosis. Therefore, strategies to improve T-cell survival and persistence are required. Mesenchymal stem/stromal cells (MSCs) exhibit immunoregulatory and tissue-restoring potential. We have previously shown that the transfer of umbilical cord MSC (UC-MSC)-derived mitochondrial (MitoT) prompts the genetic reprogramming of CD3+ T cells towards a Treg cell lineage. The potency of T cells plays an important role in effective immunotherapy, underscoring the need for improving their metabolic fitness. In the present work, we evaluate the effect of MitoT on apoptotis of native T lymphocytes and engineered CAR-T cells.
We used a cell-free approach using artificial MitoT (Mitoception) of UC-MSC derived MT to peripheral blood mononuclear cells (PBMCs) followed by RNA-seq analysis of CD3+ MitoTpos and MitoTneg sorted cells. Target cell apoptosis was induced with Staurosporine (STS), and cell viability was evaluated with Annexin V/7AAD and TUNEL assays. Changes in apoptotic regulators were assessed by flow cytometry, western blot, and qRT-PCR. The effect of MitoT on 19BBz CAR T-cell apoptosis in response to electroporation with a non-viral transposon-based vector was assessed with Annexin V/7AAD.
Gene expression related to apoptosis, cell death and/or responses to different stimuli was modified in CD3+ T cells after Mitoception. CD3+MitoTpos cells were resistant to STS-induced apoptosis compared to MitoTneg cells, showing a decreased percentage in apoptotic T cells as well as in TUNEL+ cells. Additionally, MitoT prevented the STS-induced collapse of the mitochondrial membrane potential (MMP) levels, decreased caspase-3 cleavage, increased BCL2 transcript levels and BCL-2-related BARD1 expression in FACS-sorted CD3+ T cells. Furthermore, UC-MSC-derived MitoT reduced both early and late apoptosis in CAR-T cells following electroporation, and exhibited an increasing trend in cytotoxic activity levels.
Artificial MitoT prevents STS-induced apoptosis of human CD3+ T cells by interfering with the caspase pathway. Furthermore, we observed that MitoT confers protection to apoptosis induced by electroporation in MitoTpos CAR T-engineered cells, potentially improving their metabolic fitness and resistance to environmental stress. These results widen the physiological perspective of organelle-based therapies in immune conditions while offering potential avenues to enhance CAR-T treatment outcomes where their viability is compromised.
© 2024. The Author(s).
-
Homo sapiens (Human)
-
Cell Biology
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
Improving Reliability of Immunological Assays by Defining Minimal Criteria for Cell Fitness.
In ImmunoHorizons on 1 September 2024 by Ivison, S., Boucher, G., et al.
Human PBMC-based assays are often used as biomarkers for the diagnosis and prognosis of disease, as well as for the prediction and tracking of response to biological therapeutics. However, the development and use of PBMC-based biomarker assays is often limited by poor reproducibility. Complex immunological assays can be further complicated by variation in cell handling before analysis, especially when using cryopreserved cells. Variation in postthaw viability is further increased if PBMC isolation and cryopreservation are done more than a few hours after collection. There is currently a lack of evidence-based standards for the minimal PBMC viability or "fitness" required to ensure the integrity and reproducibility of immune cell-based assays. In this study, we use an "induced fail" approach to examine the effect of thawed human PBMC fitness on four flow cytometry-based assays. We found that cell permeability-based viability stains at the time of thawing did not accurately quantify cell fitness, whereas a combined measurement of metabolic activity and early apoptosis markers did. Investigation of the impact of different types and levels of damage on PBMC-based assays revealed that only when cells were >60-70% live and apoptosis negative did biomarker values cease to be determined by cell fitness rather than the inherent biology of the cells. These data show that, to reproducibly measure immunological biomarkers using cryopreserved PBMCs, minimal acceptable standards for cell fitness should be incorporated into the assay protocol.
Copyright © 2024 The Authors.
-
Immunology and Microbiology
The immunopathological landscape of human pre-TCRα deficiency: From rare to common variants.
In Science on 1 March 2024 by Materna, M., Delmonte, O. M., et al.
We describe humans with rare biallelic loss-of-function PTCRA variants impairing pre-α T cell receptor (pre-TCRα) expression. Low circulating naive αβ T cell counts at birth persisted over time, with normal memory αβ and high γδ T cell counts. Their TCRα repertoire was biased, which suggests that noncanonical thymic differentiation pathways can rescue αβ T cell development. Only a minority of these individuals were sick, with infection, lymphoproliferation, and/or autoimmunity. We also report that 1 in 4000 individuals from the Middle East and South Asia are homozygous for a common hypomorphic PTCRA variant. They had normal circulating naive αβ T cell counts but high γδ T cell counts. Although residual pre-TCRα expression drove the differentiation of more αβ T cells, autoimmune conditions were more frequent in these patients compared with the general population.
-
FC/FACS
In mBio on 31 October 2023 by Mellergaard, M., Skovbakke, S. L., et al.
Therapies that target and aid the host immune defense to repel cancer cells or invading pathogens are rapidly emerging. Antibiotic resistance is among the largest threats to human health globally. Staphylococcus aureus (S. aureus) is the most common bacterial infection, and it poses a challenge to the healthcare system due to its significant ability to develop resistance toward current available therapies. In long-term infections, S. aureus further adapt to avoid clearance by the host immune defense. In this study, we discover a new interaction that allows S. aureus to avoid elimination by the immune system, which likely supports its persistence in the host. Moreover, we find that blocking the specific receptor (PD-1) using antibodies significantly relieves the S. aureus-imposed inhibition. Our findings suggest that therapeutically targeting PD-1 is a possible future strategy for treating certain antibiotic-resistant staphylococcal infections.
-
Immunology and Microbiology
In Front Oncol on 21 June 2023 by Pearce, D. R., Akarca, A. U., et al.
Fig.3.B

-
FC/FACS
-
Collected and cropped from Front Oncol by CiteAb, provided under a CC-BY license
Image 1 of 3
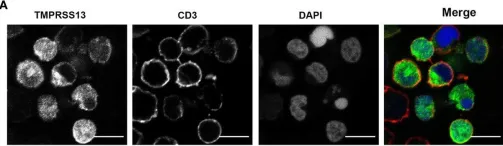
In Front Immunol on 23 September 2022 by Gatineau, J., Nidercorne, C., et al.
Fig.5.A

-
ICC-IF
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 3
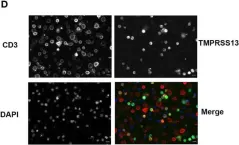
In Front Immunol on 23 September 2022 by Gatineau, J., Nidercorne, C., et al.
Fig.5.D

-
ICC-IF
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 3