Humanized immune system (HIS) mice are powerful tools for studying human immune system function and dysfunction and developing human-specific immunotherapeutics. The availability of sophisticated super immunodeficient mouse strains has allowed immune system humanization using transplants of human peripheral blood mononuclear cells (PBMC) or hematopoietic stem cells. HIS mice are used extensively in immune-oncology, while there are fewer studies in autoimmunity, especially multiple sclerosis (MS). Using the protocol described here, we generated HIS mice that show key features of MS not represented in other widely used MS models [1]. Severely immunodeficient NOD.Cg-B2mem1Tac Prkdcscid Il2rgtm1Sug /JicTac (B2m-NOG) mice, which lack murine B, T, and NK cells and murine major histocompatibility class I molecules and have defective innate immune responses, were transplanted with PBMC from HLA-DRB1-genotyped MS patients and healthy donors. Mice were successfully engrafted with hCD4 and hCD8 T and B lymphocytes and developed both spontaneous and experimental autoimmune encephalomyelitis (EAE)-enhanced T-cell lesions in the central nervous system. B-cell engraftment was highest in mice receiving cells from MS patients with serological evidence for Epstein-Barr virus (EBV) reactivation. This humanized MS model shows advantages over EAE, particularly spontaneous hCD8 T-cell lesions in the brain and spinal cord, mixed hCD8/hCD4 T-cell lesions in EAE-immunized mice, and more severe lesions in mice engrafted with PBMC from MS donors carrying the DRB1*15 MS susceptibility allele compared to DRB1*15-positive healthy and DRB1*13-positive MS donors. MS HIS mice represent simple and rapid tools for investigating human immunopathology and the efficacy of therapeutics at a personalized level. Key features • Humanization of severely immunodeficient B2m-NOG mice with human PBMC. • Engraftment analysis of human immune system in mice using multicolor flow cytometry. • Animal familiarization and handling techniques. • Epstein-Barr virus latency evaluation in human plasma. • Ex vivo characterization of engrafted T-cell cytokine responses.
©Copyright : © 2025 The Authors; This is an open access article under the CC BY license.
Product Citations: 182
PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement.
In Bio-protocol on 20 May 2025 by Dagkonaki, A., Papazian, I., et al.
-
Immunology and Microbiology
Immunogenomic profiling of circulating T cells in pediatric cancer patients during standard-of-care
Preprint on BioRxiv : the Preprint Server for Biology on 13 January 2025 by Nabbi, A., Jiang, Y., et al.
While pediatric cancer patients receive intensive chemotherapy, its impact on peripheral T cells and subsequently to disease outcomes are not fully characterized. Here, we assessed T-cell dynamics during treatment, identifying associations with outcomes through immune phenotyping and T-cell Receptor (TCR) sequencing in pediatric solid and hematologic malignancies. We show that while levels of immune checkpoint proteins (PD-1, LAG3, and TIM3) at baseline were highest in lymphomas compared to other cancer groups, they increased significantly in response to therapy in all cancers. Levels of Central Memory (CM) T cells increased in leukemias and solid tumors, while naïve T cells and cell-free TCR diversity decreased in lymphomas. By combining immune cell and TCR repertoire features across all timepoints, we proposed the Dynamic Immunogenomic Score (DIS) to measure patient-specific effects of therapy on the peripheral T-cell population. Higher DIS was associated with high-risk cancer types and logistic regression analysis revealed it may predict incidence of relapse in leukemia patients. TCR specificity analysis revealed patient-specific clonal dynamics and differential detection of virally-associated TCRs in cancer patients compared to healthy individuals. Our results highlight the potential of early upfront immunogenomic profiling in identifying high-risk patients that may be predictive in light of emerging cellular immunotherapies.
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
In vivo HIV-1 nuclear condensates safeguard against cGAS and license reverse transcription.
In The EMBO Journal on 1 January 2025 by Ay, S., Burlaud-Gaillard, J., et al.
Entry of viral capsids into the nucleus induces the formation of biomolecular condensates called HIV-1 membraneless organelles (HIV-1-MLOs). Several questions remain about their persistence, in vivo formation, composition, and function. Our study reveals that HIV-1-MLOs persisted for several weeks in infected cells, and their abundance correlated with viral infectivity. Using an appropriate animal model, we show that HIV-1-MLOs were formed in vivo during acute infection. To explore the viral structures present within these biomolecular condensates, we used a combination of double immunogold labeling, electron microscopy and tomography, and unveiled a diverse array of viral core structures. Our functional analyses showed that HIV-1-MLOs remained stable during treatment with a reverse transcriptase inhibitor, maintaining the virus in a dormant state. Drug withdrawal restored reverse transcription, promoting efficient virus replication akin to that observed in latently infected patients on antiretroviral therapy. However, when HIV-1 MLOs were deliberately disassembled by pharmacological treatment, we observed a complete loss of viral infectivity. Our findings show that HIV-1 MLOs shield the final reverse transcription product from host immune detection.
© 2024. The Author(s).
-
Biochemistry and Molecular biology
shRNA-mediated gene silencing of HDAC11 empowers CAR-T cells against prostate cancer.
In Frontiers in Immunology on 5 June 2024 by Zhang, H., Yao, J., et al.
Epigenetic mechanisms are involved in several cellular functions, and their role in the immune system is of prime importance. Histone deacetylases (HDACs) are an important set of enzymes that regulate and catalyze the deacetylation process. HDACs have been proven beneficial targets for improving the efficacy of immunotherapies. HDAC11 is an enzyme involved in the negative regulation of T cell functions. Here, we investigated the potential of HDAC11 downregulation using RNA interference in CAR-T cells to improve immunotherapeutic outcomes against prostate cancer. We designed and tested four distinct short hairpin RNA (shRNA) sequences targeting HDAC11 to identify the most effective one for subsequent analyses. HDAC11-deficient CAR-T cells (shD-NKG2D-CAR-T) displayed better cytotoxicity than wild-type CAR-T cells against prostate cancer cell lines. This effect was attributed to enhanced activation, degranulation, and cytokine release ability of shD-NKG2D-CAR-T when co-cultured with prostate cancer cell lines. Our findings reveal that HDAC11 interference significantly enhances CAR-T cell proliferation, diminishes exhaustion markers PD-1 and TIM3, and promotes the formation of T central memory TCM populations. Further exploration into the underlying molecular mechanisms reveals increased expression of transcription factor Eomes, providing insight into the regulation of CAR-T cell differentiation. Finally, the shD-NKG2D-CAR-T cells provided efficient tumor control leading to improved survival of tumor-bearing mice in vivo as compared to their wild-type counterparts. The current study highlights the potential of HDAC11 downregulation in improving CAR-T cell therapy. The study will pave the way for further investigations focused on understanding and exploiting epigenetic mechanisms for immunotherapeutic outcomes.
Copyright © 2024 Zhang, Yao, Ajmal, Farooq and Jiang.
-
Cancer Research
-
Immunology and Microbiology
In Cell Chemical Biology on 18 April 2024 by Emert-Sedlak, L. A., Tice, C. M., et al.
The HIV-1 Nef accessory factor enhances the viral life cycle in vivo, promotes immune escape of HIV-infected cells, and represents an attractive antiretroviral drug target. However, Nef lacks enzymatic activity and an active site, complicating traditional occupancy-based drug development. Here we describe the development of proteolysis targeting chimeras (PROTACs) for the targeted degradation of Nef. Nef-binding compounds, based on an existing hydroxypyrazole core, were coupled to ligands for ubiquitin E3 ligases via flexible linkers. The resulting bivalent PROTACs induced formation of a ternary complex between Nef and the cereblon E3 ubiquitin ligase thalidomide-binding domain in vitro and triggered Nef degradation in a T cell expression system. Nef-directed PROTACs efficiently rescued Nef-mediated MHC-I and CD4 downregulation in T cells and suppressed HIV-1 replication in donor PBMCs. Targeted degradation is anticipated to reverse all HIV-1 Nef functions and may help restore adaptive immune responses against HIV-1 reservoir cells in vivo.
Copyright © 2024 Elsevier Ltd. All rights reserved.
-
Immunology and Microbiology
In Nat Commun on 22 June 2023 by Nunes-Santos, C. J., Kuehn, H. S., et al.
Fig.7.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 2
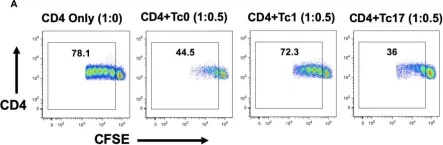
In Front Immunol on 17 November 2020 by Renavikar, P. S., Sinha, S., et al.
Fig.1.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 2