Chimeric antigen receptor (CAR)-T therapy on acute myeloid leukemia (AML) is hindered by the absence of a suitable tumor-specific antigen. Here, we propose CD97 as a potential target for CAR-T therapy against AML based on its broader and higher expression on AML cells compared to normal hematopoietic stem and progenitor cells (HSPCs). To resolve the fratricide problem caused by CD97 expression on T cells, we knock out CD97 in CAR-T cells using CRISPR-Cas9. Our CD97KO CAR-T cells eliminate both AML cell lines and primary AML cells effectively while showing tolerable toxicity to HSPCs. Furthermore, we mutate the CD3ζ domain of the CAR and find that the optimized CD97 CAR-T cells exhibit persistent anti-tumor activity both in vitro and in multiple xenograft models. Mechanistically, transcriptional profiles reveal that the optimized CAR-T cells delay differentiation and resist exhaustion. Collectively, our study supports CD97 as a promising target for CAR-T therapy against AML.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Product Citations: 132
In Cell Reports Medicine on 17 June 2025 by Shang, K., Huang, D., et al.
-
Cancer Research
-
Immunology and Microbiology
In The Journal of Biological Chemistry on 30 May 2025 by Zhang, L., Zhong, J., et al.
Fusion oncogene MLL-AF9 initiates AML via downstream targets such as HOXA9. Drivers in the complicated settings of advanced AML, however, remain to be incompletely elucidated. Any factors to incur upregulation of the effector HOXA9 predictably aggravate the effect of DOT1L-mediated H3K79 methylation on HOXA9 expression in MLL-AF9-driven AML. In the present study, we identified that SET and MYND domain-containing protein 3 (SMYD3) was overexpressed in AML and predicted a poor prognosis for patients with AML. Given that H3K4me3 typically activates the transcription of oncogenes, we hypothesized that SMYD3-catalyzed H3K4me3 may directly increase HOXA9 transcription, offering an additional regulation layer to HOXA9 gene transcription activation in MLL-AF9 AML. We tested this hypothesis and unveiled that SMYD3 is responsible for mediating H3K4me3 enrichment and for independently activating HOXA9 transcription. Transcription factor HOXA9 in turn bound to the promoter region of SMYD3 and enhanced its transcription. The resultant vicious circle of SMYD3-H3K4me3-HOXA9 exacerbated proliferation and blocked differentiation in both AML cell lines and primary cells fractionated from patients with AML. Combinational disruption of this loop and DOT1L inhibition led to enhanced anti-leukemia activity against MLL-AF9 AML in vitro and in vivo. In conclusion, our findings may advocate the current understanding regarding the underlying mechanism and offer SMYD3 as a promising intervention target to override the complicated settings in advanced AML.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
-
Biochemistry and Molecular biology
In Frontiers in Immunology on 26 May 2025 by Bielski, P., Barczyński, J., et al.
The introduction of checkpoint immunotherapeutic agents in the last decade has revolutionized cancer treatment. Although anti-PD-1, anti-PD-L1 and anti-CTLA4 are promising therapies, many patients fail to respond or relapse due to drug resistance potentially due to redundancy of immune checkpoints. One of the ways to improve the efficacy of this cancer treatment is to target two or even three immune checkpoints. To date, the benefit of combined anti-VISTA/anti-PD-L1 therapy has been confirmed, but no one has investigated the efficacy of blocking these negative immune checkpoints with a bispecific anti-VISTA/anti-PD-L1 antibody.
In this study, the bispecific antibodies (bsAbs) were produced in three formats: symmetric (IgG-HC-scFv), asymmetric (Fab-scFv-Fc(KIH)) and 2 x scFv. The binding and blocking properties of these bispecific antibodies (bsAbs) and their efficacy compared to monotherapy and combination therapy were then determined using endometrial (RL95-2), pancreatic (PANC-1) and breast (BT-20) cancer cell lines.
The bsAbs generated in this study showed weaker binding properties to PD-1 and VISTA in ELISA (EC50) than the parent antibodies (atezolizumab and onvatilimab). Blockade of VISTA/VSIG-3 binding was also weaker with bsAbs compared to onvatilimab, but the ability to block the PD-1/PD-L1 pathway was slightly better than with atezolizumab. The Fc-based bsAbs showed statistically significant higher levels of lysis of endometrial, breast and pancreatic cancer cells. The symmetric bsAbs (IgG-HC-scFv) showed the most promising therapeutic potential. Higher levels of cancer cell lysis were associated with higher levels of pro-inflammatory cytokines. Both the asymmetric and symmetric bsAbs resulted in higher secretion levels of IFN-γ, TNFα and Granzyme B than anti-VISTA, anti-PD-L1 monotherapy and anti-VISTA/anti-PD-L1 combination therapy.
The high level of tumor cell lysis and increased expression of pro-inflammatory cytokines induced by the Fc-based bsAbs suggest a novel approach for the treatment of pancreatic, endometrial and breast cancer.
Copyright © 2025 Bielski, Barczyński, Mikitiuk, Myrcha, Rykała, Boon, Gąsior, Hec-Gałązka, Holak and Sitar.
-
Cancer Research
-
Immunology and Microbiology
In BMC Immunology on 24 May 2025 by Sheng, W., Ding, Y., et al.
The identification of affordable and easily accessible indicators to predict overall survival is important for tumor immunotherapy. Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells, which promote tumor immune escape in the tumor microenvironment (TME). This study aimed to determine whether peripheral blood MDSCs could determine their potential as predictors of survival in tumor patients with immunotherapy.
Flow cytometry was used to detect peripheral blood monocytic myeloid-derived suppressor cells (M-MDSCs) and granulocytic myeloid-derived suppressor cells (G-MDSCs) in 126 patients. Multivariate Cox regression analysis was conducted to examine the associations between peripheral blood MDSCs and patient survival. The receiver operating characteristic (ROC) curve determined the optimal cutoff value for peripheral blood MDSCs and grouped the indicators. The relationship between peripheral blood M-MDSCs and the prognosis and treatment outcome of tumor patients was explored.
The proportion of peripheral blood M-MDSCs was associated with the prognosis of patients with tumors, as were tumor metastasis, the red blood cell count, absolute neutrophil count, absolute monocyte count, and BMI. Multivariate Cox regression analysis revealed that M-MDSCs, absolute lymphocyte value, and tumor metastasis were independent risk factors affecting the prognosis of patients with tumors. Detection of peripheral blood M-MDSCs obtained high sensitivity and specificity for tumor diagnosis. Patients with high M-MDSCs percentage demonstrated reduced survival durations and diminished responses to immunotherapy compared to those with low M-MDSCs percentage.
Peripheral blood M-MDSCs may be used to predict overall survival and immunotherapy efficacy outcomes. This study provides a putative predictive biomarker for clinicians to choose from to predict tumor patients' survival and the selection of receiving immunotherapy regimens.
© 2025. The Author(s).
-
FC/FACS
-
Homo sapiens (Human)
-
Cancer Research
-
Cardiovascular biology
-
Immunology and Microbiology
Systematic discovery of single-cell protein networks in cancer with Shusi
Preprint on BioRxiv : the Preprint Server for Biology on 28 April 2025 by Zhang, T., Yu, J., et al.
Context-specific protein-protein interaction (PPI) drive heterogeneity of primary tumor, forming a formidable challenge to effective cancer therapy. However, systematically mapping and modeling these interactions at single-cell resolution across diverse cancer types remains an unmet need. Here, we present Shusi, a large language model-enhanced variational graph auto-encoder model trained on over 75,010 single-cell PPI networks across 23 cancer types, to predict context-specific PPIs. Shusi outperforms existing state-of-the-art methods, as validated through orthogonal experimental evidence. Cancer-specific mutations are significantly enriched in Shusi-predicted networks, offering complementary insights to conventional marker gene-based approaches. Through systematic evaluations, we demonstrate strong associations between Shusi-predicted network topologies, genetic vulnerabilities, and therapeutic sensitivity. Finally, in acute myeloid leukemia (AML), a blood cancer where cell-state heterogeneity drives clinical resistance, Shusi pinpointed JAK2 and SHP1 as actionable vulnerabilities of resistant leukemia subpopulations, as validated experimentally in primary AML. Shusi offers a deep-learning tool for implementing precision medicine based on single-cell protein network architecture.
-
Cancer Research
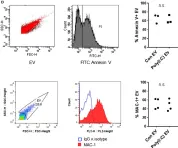
In Front Immunol on 13 February 2018 by Frank-Bertoncelj, M., Pisetsky, D. S., et al.
Fig.1.D

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 6
In PLoS One on 15 August 2014 by Lee, S. J., Choi, E. K., et al.
Fig.1.C

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 6
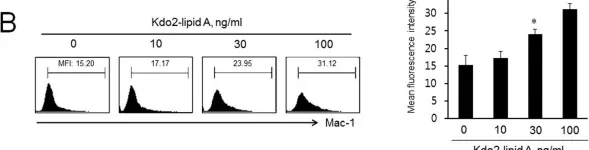
In PLoS One on 15 August 2014 by Lee, S. J., Choi, E. K., et al.
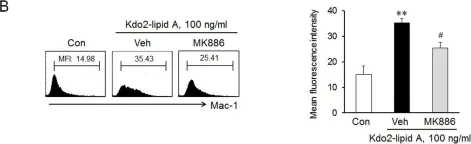
Fig.1.B

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 6
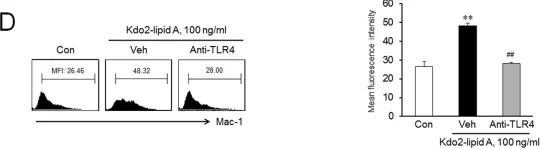
In PLoS One on 15 August 2014 by Lee, S. J., Choi, E. K., et al.
Fig.1.D

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 6
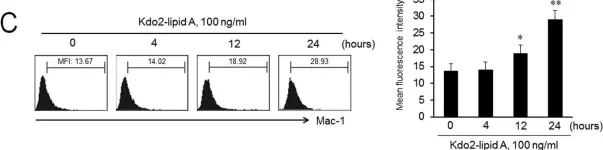
In PLoS One on 15 August 2014 by Lee, S. J., Choi, E. K., et al.
Fig.4.B

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 6
In Immun Ageing on 28 March 2009 by Gasparoto, T. H., Vieira, N. A., et al.
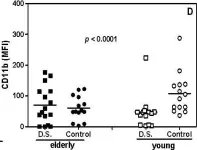
Fig.2.D

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Immun Ageing by CiteAb, provided under a CC-BY license
Image 1 of 6