Myelodysplastic syndromes (MDS) represent a heterogeneous group of clonal hematopoietic disorders characterized by ineffective haematopoiesis, refractory cytopenia, and an increased risk of progression to acute myeloid leukaemia. This study investigates the presence of cellular senescence in bone marrow (BM) CD235a+ erythrocyte precursors of MDS patients and explores its correlation with anaemia.
We assessed senescence-related markers and cell cycle distribution in BM CD235a+ erythrocyte precursors of MDS patients. Correlation analyses were conducted between the relative mRNA expression of p16INK4A, a key senescence regulator, and peripheral blood parameters.
MDS patients exhibited heightened cellular senescence characterized by increased SA-β-gal positivity, elevated p16INK4A and p21CIP1 expression, reduced CyclinD1 levels, and elevated IL-6. Cell cycle analysis revealed G0/G1 phase arrest. Correlation analysis established a negative association between p16INK4A expression and reticulocyte count, RBC count, haemoglobin concentration, indicating a direct link between BM erythrocyte precursors senescence and anaemia severity.
MDS patients have accelerated senescence of bone marrow erythrocyte precursors, which is related to their anaemia. The observed correlation underscores the potential significance of senescence-targeted interventions in managing anaemia in MDS.Key MessagesBone marrow CD235a⁺ erythroid precursors in MDS patients exhibit accelerated senescence, characterized by cell cycle arrest and increased inflammatory markers. p16INK4A expression negatively correlates with anaemia severity, suggesting senescence as a key contributor to MDS-related anaemia.
Product Citations: 308
Accelerated senescence of bone marrow erythrocyte precursors in myelodysplastic syndrome.
In Annals of Medicine on 1 December 2025 by Liu, M., Yang, L., et al.
Systematic discovery of single-cell protein networks in cancer with Shusi
Preprint on BioRxiv : the Preprint Server for Biology on 28 April 2025 by Zhang, T., Yu, J., et al.
Context-specific protein-protein interaction (PPI) drive heterogeneity of primary tumor, forming a formidable challenge to effective cancer therapy. However, systematically mapping and modeling these interactions at single-cell resolution across diverse cancer types remains an unmet need. Here, we present Shusi, a large language model-enhanced variational graph auto-encoder model trained on over 75,010 single-cell PPI networks across 23 cancer types, to predict context-specific PPIs. Shusi outperforms existing state-of-the-art methods, as validated through orthogonal experimental evidence. Cancer-specific mutations are significantly enriched in Shusi-predicted networks, offering complementary insights to conventional marker gene-based approaches. Through systematic evaluations, we demonstrate strong associations between Shusi-predicted network topologies, genetic vulnerabilities, and therapeutic sensitivity. Finally, in acute myeloid leukemia (AML), a blood cancer where cell-state heterogeneity drives clinical resistance, Shusi pinpointed JAK2 and SHP1 as actionable vulnerabilities of resistant leukemia subpopulations, as validated experimentally in primary AML. Shusi offers a deep-learning tool for implementing precision medicine based on single-cell protein network architecture.
-
Cancer Research
In Frontiers in Cell and Developmental Biology on 12 March 2025 by Verma, R., Kailashiya, J., et al.
Prion diseases are neurodegenerative disorders where infectious prion proteins (PrP) featuring an amyloidogenic amino acid sequence, PrP (106-126), accumulate in the brain leading to neuroinflammation while it can also access circulation by breaching the blood-brain barrier. Platelets are highly sensitive cells in blood, which have been widely employed as "peripheral" model for neurons. In addition to their stellar roles in hemostasis and thrombosis, platelets are also known to function as immune cells and possess necessary components of functional inflammasome. This study demonstrates that prion proteins drive inflammasome assembly in platelets and upregulate activity of caspase-1, a critical readout of functional inflammasomes.
Flow cytometric analysis was performed to measure intracellular ROS levels, caspase-1 activity, and platelet-monocyte/neutrophil interactions. Microscopy was used to assess the co-localization of NLRP3 and ASC.
Inflammasome activation is fuelled by reactive oxygen species (ROS) generated in prion-stimulated platelets that eventually leads to formation of platelet-monocyte/neutrophil aggregates, which was prohibited by small-molecule inhibitors of either caspase-1 or ROS.
Thus, in addition to their neurotoxic effects on neuronal cells and stimulation of pro-coagulant activity in platelets, prions also unleash an inflammatory response in the organism.
Copyright © 2025 Verma, Kailashiya, Mukherjee, Chaurasia and Dash.
-
Immunology and Microbiology
In Bone Research on 24 February 2025 by Li, Z., Jiang, J., et al.
Knee arthrofibrosis, characterized by excessive matrix protein production and deposition, substantially impairs basic daily functions, causing considerable distress and financial burden. However, the underlying pathomechanisms remain unclear. Here, we characterized the heterogeneous cell populations and cellular pathways by combination of flow cytometry and single-cell RNA-seq analysis of synovial tissues from six patients with or without knee arthrofibrosis. Increased macrophages and fibroblasts were observed with decreased numbers of fibroblast-like synoviocytes, endothelial cells, vascular smooth muscle cells, and T cells in the arthrofibrosis group compared with negative controls. Notably, fibroblasts were discovered to interact with macrophages, and lead to fibrosis through TGF-β pathway induced CCN2 expression in fibroblasts. CCN2 was demonstrated to be required for fibroblast pro-fibrotic functions (activation, proliferation, and migration) through TGFBR/SMAD pathway. The expression of CCN2 was positively correlated with the collagen volume and TGF-β expression and negatively associated with patient-reported outcome measures in another cohort of patients with knee arthrofibrosis. Our study reveals the role of CCN2 in the fibroblast-macrophage interaction through TGF-β pathway which might help to shed light on CCN2 as a potential biomarker.
© 2025. The Author(s).
-
FC/FACS
-
Homo sapiens (Human)
-
Genetics
-
Immunology and Microbiology
Temperature-based MHC class-I multimer peptide exchange for human HLA-A, B and C
Preprint on BioRxiv : the Preprint Server for Biology on 23 December 2024 by Pothast, C. R., Derksen, I., et al.
T cell recognition of specific antigens presented by major histocompatibility complexes class-I (MHC-I) can play an important role during immune responses against pathogens and cancer cells. Detection of T cell immunity is based on assessing the presence of antigen-specific cytotoxic CD8+ T cells using MHC class-I (MHC-I) multimer technology. Previously we have designed conditional peptides for HLA-A*02:01, H-2K b and HLA-E that form stable peptide-MHC-I-complexes at low temperatures and dissociate when exposed to a defined elevated temperature. The resulting conditional MHC-I complex can easily and without additional handling be exchanged with a peptide of interest, allowing to exchange peptides in a ready-to-use multimer and a high-throughput manner. Here we present data that this peptide-exchange technology is a general applicable, ready-to-use and fast approach to load many different peptides in MHC-I multimers for alleles of the HLA-A, HLA-B and HLA-C loci. We describe the development of conditional peptides for HLA-A*03:01, HLA-A*11:01, HLA-B*07:02 and HLA-C*07:02 that only form stable peptide-MHC-I complexes at low temperatures, allowing peptide exchange at higher defined temperature. We document the ease and flexibility of this technology by monitoring CD8+ T cell responses to virus-specific peptide-MHC complexes in patients. Graphical abstract Highlights T cell immunity relies on antigen-specific CD8+ T cells recognizing peptide MHC-I complexes. Establishing temperature-based peptide exchange across multiple HLA alleles, resulting in a robust, easy, and fast system to generate peptide MHC-I complexes. Temperature-based MHC class-I multimer demonstrate applicability across major MHC-I gene families for monitoring CD8+ T cell responses. Easy high-throughput peptide exchange potential, enhancing clinical utility of MHC multimer technology.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
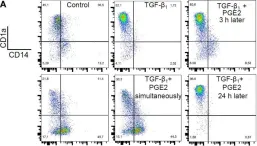
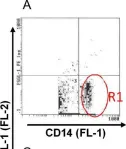
In Front Immunol on 11 July 2018 by Remes Lenicov, F., Paletta, A. L., et al.
Fig.8.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 8
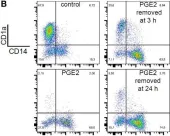
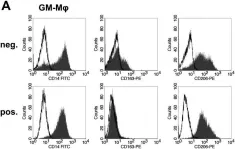
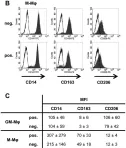
In Front Immunol on 11 July 2018 by Remes Lenicov, F., Paletta, A. L., et al.
Fig.8.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 8
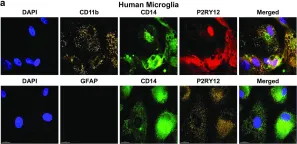
In J Neurovirol on 1 February 2017 by García-Mesa, Y., Jay, T. R., et al.
Fig.6.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from J Neurovirol by CiteAb, provided under a CC-BY license
Image 1 of 8
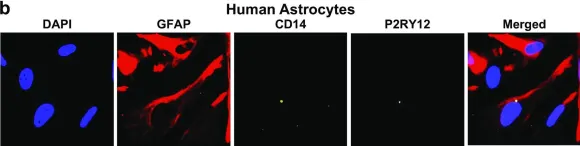
In J Neurovirol on 1 February 2017 by García-Mesa, Y., Jay, T. R., et al.
Fig.5.A

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from J Neurovirol by CiteAb, provided under a CC-BY license
Image 1 of 8
In J Neurovirol on 1 February 2017 by García-Mesa, Y., Jay, T. R., et al.
Fig.5.B

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from J Neurovirol by CiteAb, provided under a CC-BY license
Image 1 of 8
In Glycobiology on 1 October 2016 by Kanabar, V., Tedaldi, L., et al.
Fig.2.A

-
FC/FACS
-
Collected and cropped from Glycobiology by CiteAb, provided under a CC-BY license
Image 1 of 8
In PLoS One on 19 June 2013 by Neu, C., Sedlag, A., et al.
Fig.1.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 8
In PLoS One on 19 June 2013 by Neu, C., Sedlag, A., et al.
Fig.1.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 8