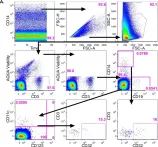
Mesenchymal stem cells (MSCs) are frequently used for therapeutic applications in both pre-clinical and clinical settings owing to their capacity for immune modulation and neuroprotective effects. However, transient fever is commonly observed as an adverse event following MSC injection in patients with Alzheimer's disease (AD). In this study, we investigated the potential impact of immunosuppressants such as dexamethasone and tacrolimus on altering the characteristics of human mesenchymal stem cells (hMSCs). Additionally, we examined whether these immunosuppressants affect the persistence of hMSCs or the immune response upon their administration into the brain parenchyma of AD mice. The exposure of hMSCs to high concentrations of dexamethasone and tacrolimus in vitro did not significantly alter the characteristics of hMSCs. The expression of genes related to innate immune responses, such as Irak1, Irf3, Nod1, and Ifnar1, was significantly downregulated by the additional administration of dexamethasone and tacrolimus to the brain parenchyma of AD mice. However, hMSC persistence in the AD mouse brain was not affected. The results of this study support the use of immunosuppressants to mitigate fever during stem cell therapy in patients with AD.
Product Citations: 189
In International Journal of Stem Cells on 30 May 2025 by Lee, N. K., Na, D. L., et al.
-
Stem Cells and Developmental Biology
In Journal of Endocrinological Investigation on 1 May 2025 by Zhang, X., van Greevenbroek, M. M. J., et al.
Elevated methylglyoxal (MGO) levels and altered immune cell responses are observed in diabetes. MGO is thought to modulate immune cell activation. The current study investigated whether fasting or post-glucose-load plasma MGO concentrations are associated with circulating immune cell counts and activation in a large cohort study.
696 participants of The Maastricht Study (age 60.3 ± 8.4 years, 51.9% women) underwent an oral glucose tolerance test (OGTT). Fasting and post-OGTT plasma MGO concentrations were measured using mass spectrometry. Numbers and activation of circulating immune cells at fasting state were quantified using flow cytometry. Activation scores were calculated by averaging individual marker z-scores for neutrophils (CD11b, CD11c, CD16) and classical, intermediate, and non-classical monocytes (CD11b, CD11c, CX3XR1, HLA-DR). Associations were analysed using multiple linear regression adjusted for potential confounders. Stratified analyses were performed for glucose metabolism status for associations between plasma MGO levels and immune cell counts.
Higher fasting plasma MGO concentrations were significantly associated with higher numbers of intermediate (β = 0.09 [95%CI 0.02; 0.17]) and non-classical monocytes (0.08 [0.002; 0.15]), but with lower activation scores for the intermediate monocytes (-0.14 [-0.22; -0.06]). Stratified analyses showed that positive associations between fasting plasma MGO levels and numbers of intermediate and non-classical monocytes appear only in participants with type 2 diabetes. Post-OGTT plasma MGO concentrations were not consistently associated with immune cells counts or activation.
Higher fasting plasma MGO concentrations are associated with higher intermediate and non-classical monocyte counts but with lower activation of intermediate monocytes.
© 2025. The Author(s).
-
Homo sapiens (Human)
Temperature-based MHC class-I multimer peptide exchange for human HLA-A, B and C
Preprint on BioRxiv : the Preprint Server for Biology on 23 December 2024 by Pothast, C. R., Derksen, I., et al.
T cell recognition of specific antigens presented by major histocompatibility complexes class-I (MHC-I) can play an important role during immune responses against pathogens and cancer cells. Detection of T cell immunity is based on assessing the presence of antigen-specific cytotoxic CD8+ T cells using MHC class-I (MHC-I) multimer technology. Previously we have designed conditional peptides for HLA-A*02:01, H-2K b and HLA-E that form stable peptide-MHC-I-complexes at low temperatures and dissociate when exposed to a defined elevated temperature. The resulting conditional MHC-I complex can easily and without additional handling be exchanged with a peptide of interest, allowing to exchange peptides in a ready-to-use multimer and a high-throughput manner. Here we present data that this peptide-exchange technology is a general applicable, ready-to-use and fast approach to load many different peptides in MHC-I multimers for alleles of the HLA-A, HLA-B and HLA-C loci. We describe the development of conditional peptides for HLA-A*03:01, HLA-A*11:01, HLA-B*07:02 and HLA-C*07:02 that only form stable peptide-MHC-I complexes at low temperatures, allowing peptide exchange at higher defined temperature. We document the ease and flexibility of this technology by monitoring CD8+ T cell responses to virus-specific peptide-MHC complexes in patients. Graphical abstract Highlights T cell immunity relies on antigen-specific CD8+ T cells recognizing peptide MHC-I complexes. Establishing temperature-based peptide exchange across multiple HLA alleles, resulting in a robust, easy, and fast system to generate peptide MHC-I complexes. Temperature-based MHC class-I multimer demonstrate applicability across major MHC-I gene families for monitoring CD8+ T cell responses. Easy high-throughput peptide exchange potential, enhancing clinical utility of MHC multimer technology.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
In IScience on 20 December 2024 by Yang, W., Li, L., et al.
Inflammatory cells infiltration in the cerebrospinal fluid is a hallmark of severe enterovirus 71 (EV71) infection, but which type of immune cells are critical for severe EV71 infection remains unclear. Here, we observe that both neutrophils and macrophages are increased in the brains of patients and mice with severe EV71 infection, and the depletion of neutrophils but not macrophages results in a marked enhancement of survival of EV71-infected mice. Furthermore, CCR1/3 may play an important role in CCL3 facilitating the accumulation of neutrophils in the brains of patients. Inhibition of CCL3 by anti-CCL3 antibodies or selected miRNAs significantly reduces the neutrophils infiltration in brains and the mortality of EV71-infected mice. Collectively, CCL3-mediated neutrophils recruitment into the brain contributes to the severe immunopathology of EV71 infection, which provides a potential diagnostic and therapeutic target for EV71 infection.
© 2024 The Author(s).
-
Immunology and Microbiology
In Stem Cell Research & Therapy on 12 October 2024 by Czosseck, A., Chen, M. M., et al.
Cell therapy can protect cardiomyocytes from hypoxia, primarily via paracrine secretions, including extracellular vesicles (EVs). Since EVs fulfil specific biological functions based on their cellular origin, we hypothesised that EVs from human cardiac stromal cells (CMSCLCs) obtained from coronary artery bypass surgery may have cardioprotective properties.
This study characterises CMSCLC EVs (C_EVs), miRNA cargo, cardioprotective efficacy and transcriptomic modulation of hypoxic human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs). C_EVs are compared to bone marrow mesenchymal stromal cell EVs (B_EVs) which are a known therapeutic EV type.
Cells were characterised for surface markers, gene expression and differentiation potential. EVs were compared for yield, phenotype, and ability to protect hiPSC-CMs from hypoxia/reoxygenation injury. EV dose was normalised by both protein concentration and particle count, allowing direct comparison. C_EV and B_EV miRNA cargo was profiled and RNA-seq was performed on EV-treated hypoxic hiPSC-CMs, then data were integrated by multi-omics. Confirmatory experiments were carried out using miRNA mimics.
At the same dose, C_EVs were more effective than B_EVs at protecting CM integrity, reducing apoptotic markers, and cell death during hypoxia. While C_EVs and B_EVs shared 70-77% similarity in miRNA content, C_EVs contained unique miRNAs, including miR-202-5p, miR-451a and miR-142-3p. Delivering miRNA mimics confirmed that miR-1260a and miR-202/451a/142 were cardioprotective, and the latter upregulated protective pathways similar to whole C_EVs.
This study demonstrates the potential of cardiac tissues, routinely discarded following surgery, as a valuable source of EVs for myocardial infarction therapy. We also identify miR-1260a as protective of CM hypoxia.
© 2024. The Author(s).
-
Homo sapiens (Human)
-
Cardiovascular biology
-
Stem Cells and Developmental Biology
In Mutagenesis on 4 May 2022 by Vernon, A. R., Pemberton, R. M., et al.
Fig.6.A

-
FC/FACS
-
Collected and cropped from Mutagenesis by CiteAb, provided under a CC-BY license
Image 1 of 2
In PLoS One on 11 May 2016 by Cheeseman, H. M., Carias, A. M., et al.
Fig.2.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 2