Human cytomegalovirus (HCMV) is a relevant pathogen, especially for individuals with impaired immunity. Harnessing potent immune antagonists, HCMV circumvents sterile immunity. Given that HCMV prevents the upregulation of human leukocyte antigen (HLA)-DP and HLA-DR, we screened a library of HCMV genes by co-expression with the HLA class II (HLA-II)-inducing transcription coordinator class II transactivator (CIITA). We identified the latency regulator pUS28 as an interaction factor and potent viral antagonist of CIITA-driven expression of CD74, HLA-DR, HLA-DM, HLA-DQ, and HLA-DP. Both wt-pUS28 and a mutant incapable of inducing G protein-coupled signaling (R129A), but not a mutant lacking the C-terminus, drastically reduced the CIITA protein abundance post-transcriptionally. While control CD4 + T cells from HCMV-seropositive individuals vigorously responded to CIITA-expressing cells decorated with HCMV antigens, pUS28 expression was sufficient to inhibit HLA-II induction and immune recognition by HCMV-specific CD4 + T cells. Our data uncover pUS28 to be employed by HCMV to evade HLA-II-mediated recognition by CD4 + T cells.
© 2025, Maassen et al.
Product Citations: 191
The human cytomegalovirus-encoded pUS28 antagonizes CD4+ T cell recognition by targeting CIITA.
In eLife on 3 July 2025 by Maassen, F., Le-Trilling, V. T. K., et al.
-
Immunology and Microbiology
In Haematologica on 1 July 2025 by Torun, A., Zdanowicz, A., et al.
Our investigation uncovers that nanomolar concentrations of salinomycin, monensin, nigericin, and narasin (a group of potassium/ sodium cation carriers) robustly enhance surface expression of CD20 antigen in B-cell-derived tumor cells, including primary malignant cells of chronic lymphocytic leukemia and diffuse large B-cell lymphoma. Experiments in vitro, ex vivo, and animal model reveal a novel approach of combining salinomycin or monensin with therapeutic anti-CD20 monoclonal antibodies or anti-CD20 chimeric antigen receptor T cells, significantly improving non-Hodgkin lymphoma therapy. The results of RNA sequencing, genetic editing, and chemical inhibition delineate the molecular mechanism of CD20 upregulation, at least partially, to the downregulation of MYC, the transcriptional repressor of the MS4A1 gene encoding CD20. Our findings propose the cation carriers as compounds targeting MYC oncogene, which can be combined with anti-CD20 antibodies or adoptive cellular therapies to treat non-Hodgkin lymphoma and mitigate resistance, which frequently depends on the CD20 antigen loss, offering new solutions to improve patient outcomes.
-
Homo sapiens (Human)
-
Cardiovascular biology
-
Immunology and Microbiology
Novel PAP-targeted CAR-T therapy enhances antitumor efficacy through CoupledCAR approach.
In Journal for Immunotherapy of Cancer on 31 May 2025 by Cao, Z., Pu, C., et al.
The challenges that remain in the treatment of solid tumors with chimeric antigen receptor (CAR)-T cells include limited solid tumor-specific targets and poor CAR-T cell expansion and function due to limited availability of solid tumor antigens outside the tumor microenvironment. Prostate cancer is the second most common cancer among men worldwide. Current CAR-T therapies for prostate cancer lack specific targets, posing safety risks. To overcome these problems, we identified prostatic acid phosphatase (PAP, also known as ACPP or ACP3) as a feasible CAR-T target for prostate cancer and developed CoupledCAR, a novel approach for expanding tumor-targeting CAR-T cells without tumor antigens.
We analyzed the expression of PAP from The Cancer Genome Atlas database and validated its expression in normal and cancer tissues through immunohistochemistry staining. To generate anti-PAP specific antibodies, we screened the human single-chain antibody library using transmembrane PAP-His antigen and selected antibodies based on their binding ability and specificity. We constructed PAP-targeted CAR and evaluated their antitumor efficacy both in vitro and in vivo. We validated the function of PAP CoupledCAR in both in vitro and in vivo experiments, and further analyzed its mechanism using single-cell RNA sequencing (scRNA-Seq).
PAP was specifically expressed in prostate epithelial and prostate cancer cells, with no expression in other tissues. Seven single-chain variable fragments were screened from the human single-chain antibody library, with S5D1 showing the highest binding ability for the PAP. PAP CAR-T cells demonstrated strong antitumor efficacy both in vitro and in vivo. Furthermore, the CoupledCAR system significantly expanded PAP CAR-T cells, promoting memory-like status, reducing exhaustion, and enhancing their antitumor efficacy. The scRNA-Seq demonstrated that the expansion of PAP CAR-T cells in the CoupledCAR system is mediated by costimulatory signals and cytokine signals, rather than T-cell receptor signals.
Our study is the first to demonstrate that PAP is a specific target for CAR-T therapy in prostate cancer, both in vitro and in vivo. We developed the CoupledCAR platform technology for solid tumor CAR-T cell therapy, enabling the expansion of tumor-targeting CAR-T cells without requiring tumor antigens and thereby enhancing their functionality against solid tumors.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
In Respiratory Research on 15 April 2025 by Harper, R. L., Zhou, X., et al.
It is well-established that patients with pulmonary arterial hypertension (PAH) exhibit increased recruitment of circulating monocytes to their pulmonary arteries. However, it remains unclear whether these monocytes have intrinsic abnormalities that contribute to their recruitment and to PAH pathogenesis. This study aimed to characterize the gene expression profiles of circulating classical, intermediate, and non-classical monocytes and assess their maturation trajectory in patients with idiopathic (I) PAH compared to control subjects. Additionally, it sought to explore the relationship between the observed IPAH abnormalities and deficiencies in bone morphogenetic receptor 2 (BMPR2), the most frequently mutated gene in PAH, and to assess adhesion and transendothelial migration, key processes in monocyte infiltration of pulmonary arteries.
Differentially expressed genes and maturation trajectories of circulating monocytes from patients with IPAH vs. control subjects were compared using single cell RNA sequencing (scRNAseq), followed by FACS analysis. Observations from IPAH and control cells were related to reduced BMPR2 using a THP1 monocyte cell line with BMPR2 reduced by siRNA as well as induced pluripotent stem cell (iPSC) derived monocytes (iMono) from hereditary (H) PAH patients with a BMPR2 mutation and monocytes from mice with Bmpr2 deleted (MON-Bmpr2-/-).
Classical IPAH monocytes have decreased CD14 mRNA leading to a deviation in their maturation trajectory and early terminal fate, which is not rescued by cytokine treatment. Monocytes that evade early cell death show elevated STAT1, PPDPF and HLA-B, and an interferon (IFN) signature indicative of an altered activation state. A strong link between decreased BMPR2 and CD14 was observed in THP1 cells and in HPAH iMono with a BMPR2 mutation associated with STAT1 and IFN related genes, and in monocytes from MON-Bmpr2-/- mice. Increased adhesion to iPSC-derived endothelial cells (iECs) in HPAH-BMPR2 mutant iMono was associated with elevated ICAM1 expression. Enhanced transendothelial migration of these cells was associated with the reduction in endothelial VE-cadherin (CDH5).
IPAH monocytes exhibit an altered activation state associated with reduced BMPR2 and CD14, along with elevated STAT1-IFN expression. These changes are linked to intrinsic functional abnormalities that contribute to the monocytes' increased propensity to invade the pulmonary circulation.
© 2025. The Author(s).
-
FC/FACS
-
Cardiovascular biology
In Frontiers in Immunology on 11 April 2025 by Xie, S., Long, J., et al.
Anti-CD19 chimeric antigen receptor T (CAR-T) cell therapy has proven effective for treating relapsed or refractory acute B cell leukemia. However, challenges such as cytokine release syndrome, T cell dysfunction, and exhaustion persist. Enhancing CAR-T cell efficacy through changing CAR internalization and recycling is a promising approach. The transmembrane domain is the easiest motif to optimize for modulating CAR internalization and recycling without introducing additional domains, and its impact on CAR internalization and recycling has not yet been thoroughly explored. In this study, we aim to enhance CAR-T cell function by focusing on the solely transmembrane domain design.
Utilizing plasmid construction and lentivirus generation, we get two different transmembrane CAR-T cells [19CAR-T(1a) and 19CAR-T(8α)]. Through co-culture with tumor cells, we evaluate CAR dynamic change, activation levels, exhaustion markers, mitochondrial function, and differentiation in both CAR-T cells. Furthermore, immunofluorescence microscopy analysis is performed to reveal the localization of internalized CAR molecules. RNA sequencing is used to detect the transcriptome of activated CAR-T cells. Finally, a mouse study is utilized to verify the anti-tumor efficacy of 19CAR-T(1a) cells in vivo.
Our findings demonstrate that 19CAR-T(1a) has lower surface CAR expression, faster internalization, and a higher recycling rate compared to 19CAR-T(8α). Internalized 19CAR(1a) co-localizes more with early and recycling endosomes, and less with lysosomes than 19CAR(8α). These features result in lower activation levels, less cytokine release, and reduced exhaustion markers in 19CAR-T(1a). Furthermore, CAR-T cells with CD1a transmembrane domain also exhibit a superior anti-tumor ability and reduced exhaustion in vivo.
Overall, we demonstrate that the transmembrane domain plays a critical role in CAR-T cell function. An optimized transmembrane domain can alleviate cytokine release syndrome and reduce CAR-T cell exhaustion, providing a direction for CAR design to enhance CAR-T cell function.
Copyright © 2025 Xie, Long, Wang, Xiang, Xian, Wang, Dou, Zhang, Li, Kang, Chen, Zhao, Xu and Liu.
-
Immunology and Microbiology
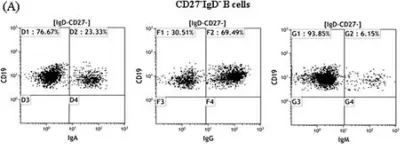
In Arthritis Res Ther on 14 March 2015 by Mahmood, Z., Muhammad, K., et al.
Fig.2.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Arthritis Res Ther by CiteAb, provided under a CC-BY license
Image 1 of 1