Abstract Sepsis-associated acute kidney injury (SA-AKI) portends severe health burden due to significant morbidity and mortality, while early diagnosis remains challenging. In this study, proximity-dependent barcoding assay (PBA) was established to profile the surface proteome of single urinary extracellular vesicle (uEV). Principle uEV clusters with unique function and origination were profiled in SA-AKI. Reduction of complement receptor CD35 on single uEV (CD35-uEV) was revealed as a novel biomarker from one of the main EV clusters with significant proportional differences. CD35-uEV demonstrated high diagnostic accuracy for SA-AKI (receiver operating characteristic-area under the curve (ROC-AUC), 0.98 in screening cohort (n=16), and 0.89 in validation cohort (n=134)). Besides, CD35-uEV enables identification of subclinical AKI (ROC-AUC, 0.84 in prospective cohort (n=72)) which was independent of other clinical parameters as validated by multivariate analysis (p<0.001). Moreover, CD35-uEV correlated closely with AKI severity which also predicts persistent AKI (ROC-AUC, 0.77), progression to AKD (ROC-AUC, 0.66), and mortality risks (ROC-AUC, 0.70). Integrative single-cell and spatial transcriptomics analysis identified that CD35-uEV originated from injured podocyte characterized with diminished CD35 expression. The combination of CD35-uEV with tubular injury biomarkers (TIMP2*IGFBP7) showed improved accuracy in identifying subclinical SA-AKI and prediction of severe stages. Overall, this study identified a novel biomarker on single uEV related to injured podocyte for early diagnosis and risk stratification of SA-AKI.
Product Citations: 32
Preprint on Research Square on 10 December 2024 by Lv, L., Li, N., et al.
In Frontiers in Veterinary Science on 3 September 2024 by Stokol, T., Thomas, S. I., et al.
CD80, a co-stimulatory molecule required for optimal T cell activation, is expressed on antigen-presenting cells, including monocytes and dendritic cells, in dogs and humans. We hypothesized that CD80 would be expressed on tumor cells in dogs from acute myeloid leukemia (AML) but not dogs with lymphoid neoplasms.
We first evaluated the cellular staining pattern of a hamster anti-murine CD80 antibody (clone 16-10A1, ThermoFisher Scientific Cat# 17-0801-82, RRID: AB_469417) in blood and bone marrow aspirates from healthy dogs. Using flow cytometric analysis and examination of modified Wright's-stained cytologic smears of unsorted and flow cytometric or immunomagnetic bead-sorted leukocytes, we show that the antibody binds to mature and immature neutrophils and monocytes, but not lymphocytes or eosinophils, in blood and bone marrow. We then added the antibody to routine flow cytometric panels for immunophenotyping hematopoietic neoplasms in dogs. We found that the antibody labeled tumor cells in 72% of 39 dogs with AML and 36% of 11 dogs with acute leukemia expressing lymphoid and myeloid markers ("mixed lineage") but none of the dogs with B (n = 37) or T (n = 35) lymphoid neoplasms. A higher proportion of tumor cells in dogs with AML were labeled with the anti-CD80 antibody vs antibodies against other myeloid-associated antigens, including CD4 (36%, p = 0.003), CD11b (44%), CD11c (46%), CD14 (38%, p = 0.006) and CD18 (59%, clone YFC118). In contrast, antibodies against CD11b and CD11c bound to tumor cells in 8-32% of the lymphoid neoplasms.
We show that CD80, as detected by antibody clone 16-10A1, is a sensitive and specific marker for AML and would be useful to include in flow cytometric immunophenotyping panels in dogs.
Copyright © 2024 Stokol, Thomas, Hoffman and Zhao.
-
Cancer Research
-
Veterinary Research
In Cell Reports on 23 July 2024 by Pelletier, A. N., Sánchez, G. P., et al.
Protective immunity to dengue virus (DENV) requires antibody response to all four serotypes. Systems vaccinology identifies a multi-OMICs pre-vaccination signature and mechanisms predictive of broad antibody responses after immunization with a tetravalent live attenuated DENV vaccine candidate (Butantan-DV/TV003). Anti-inflammatory pathways, including TGF-β signaling expressed by CD68low monocytes, and the metabolites phosphatidylcholine (PC) and phosphatidylethanolamine (PE) positively correlate with broadly neutralizing antibody responses against DENV. In contrast, expression of pro-inflammatory pathways and cytokines (IFN and IL-1) in CD68hi monocytes and primary and secondary bile acids negatively correlates with broad DENV-specific antibody responses. Induction of TGF-β and IFNs is done respectively by PC/PE and bile acids in CD68low and CD68hi monocytes. The inhibition of viral sensing by PC/PE-induced TGF-β is confirmed in vitro. Our studies show that the balance between metabolites and the pro- or anti-inflammatory state of innate immune cells drives broad and protective B cell response to a live attenuated dengue vaccine.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
FC/FACS
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
FLT3L governs the development of partially overlapping hematopoietic lineages in humans and mice.
In Cell on 23 May 2024 by Momenilandi, M., Levy, R., et al.
FMS-related tyrosine kinase 3 ligand (FLT3L), encoded by FLT3LG, is a hematopoietic factor essential for the development of natural killer (NK) cells, B cells, and dendritic cells (DCs) in mice. We describe three humans homozygous for a loss-of-function FLT3LG variant with a history of various recurrent infections, including severe cutaneous warts. The patients' bone marrow (BM) was hypoplastic, with low levels of hematopoietic progenitors, particularly myeloid and B cell precursors. Counts of B cells, monocytes, and DCs were low in the patients' blood, whereas the other blood subsets, including NK cells, were affected only moderately, if at all. The patients had normal counts of Langerhans cells (LCs) and dermal macrophages in the skin but lacked dermal DCs. Thus, FLT3L is required for B cell and DC development in mice and humans. However, unlike its murine counterpart, human FLT3L is required for the development of monocytes but not NK cells.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
FC/FACS
-
Homo sapiens (Human)
Evaluation of the Abdala Vaccine: Antibody and Cellular Response to the RBD Domain of SARS-CoV-2.
In Vaccines on 30 November 2023 by Islas-Vazquez, L., Alvarado-Alvarado, Y. C., et al.
Abdala is a recently released RBD protein subunit vaccine against SARS-CoV-2. A few countries, including Mexico, have adopted Abdala as a booster dose in their COVID-19 vaccination schemes. Despite that, most of the Mexican population has received full-scheme vaccination with platforms other than Abdala; little is known regarding Abdala's immunological features, such as its antibody production and T- and B-cell-specific response induction. This work aimed to study antibody production and the adaptive cellular response in the Mexican population that received the Abdala vaccine as a booster. We recruited 25 volunteers and evaluated their RBD-specific antibody production, T- and B-cell-activating profiles, and cytokine production. Our results showed that the Abdala vaccine increases the concentration of RBD IgG-specific antibodies. Regarding the cellular response, after challenging peripheral blood cultures with RBD, the plasmablast (CD19+CD27+CD38High) and transitional B-cell (CD19+CD21+CD38High) percentages increased significantly, while T cells showed an increased activated phenotype (CD3+CD4+CD25+CD69+ and CD3+CD4+CD25+HLA-DR+). Also, IL-2 and IFN-γ increased significantly in the supernatant of the RBD-stimulated cells. Our results suggest that Abdala vaccination, used as a booster, evokes antibody production and the activation of previously generated memory against the SARS-CoV-2 RBD domain.
-
FC/FACS
-
COVID-19
-
Immunology and Microbiology
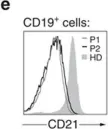
In Nat Commun on 19 November 2014 by Willmann, K. L., Klaver, S., et al.
Fig.5.E

-
FC/FACS
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 1