The discovery of upstream regulatory genes of a gene of interest still remains challenging. Here we applied a scalable computational method to unbiasedly predict candidate regulatory genes of critical transcription factors by searching the whole genome. We illustrated our approach with a case study on the master regulator FOXP3 of human primary regulatory T cells (Tregs). While target genes of FOXP3 have been identified, its upstream regulatory machinery still remains elusive. Our methodology selected five top-ranked candidates that were tested via proof-of-concept experiments. Following knockdown, three out of five candidates showed significant effects on the mRNA expression of FOXP3 across multiple donors. This provides insights into the regulatory mechanisms modulating FOXP3 transcriptional expression in Tregs. Overall, at the genome level this represents a high level of accuracy in predicting upstream regulatory genes of key genes of interest.
© 2024. The Author(s).
Product Citations: 76
In NPJ Systems Biology and Applications on 29 May 2024 by Magni, S., Sawlekar, R., et al.
-
FC/FACS
-
Immunology and Microbiology
In Nature Communications on 7 May 2024 by Prelli Bozzo, C., Laliberté, A., et al.
Innate antiviral factors are essential for effective defense against viral pathogens. However, the identity of major restriction mechanisms remains elusive. Current approaches to discover antiviral factors usually focus on the initial steps of viral replication and are limited to a single round of infection. Here, we engineered libraries of >1500 replication-competent HIV-1 constructs each expressing a single gRNAs to target >500 cellular genes for virus-driven discovery of antiviral factors. Passaging in CD4+ T cells robustly enriched HIV-1 encoding sgRNAs against GRN, CIITA, EHMT2, CEACAM3, CC2D1B and RHOA by >50-fold. Using an HIV-1 library lacking the accessory nef gene, we identified IFI16 as a Nef target. Functional analyses in cell lines and primary CD4+ T cells support that the HIV-driven CRISPR screen identified restriction factors targeting virus entry, transcription, release and infectivity. Our HIV-guided CRISPR technique enables sensitive discovery of physiologically relevant cellular defense factors throughout the entire viral replication cycle.
© 2024. The Author(s).
In mBio on 31 October 2023 by Mellergaard, M., Skovbakke, S. L., et al.
Therapies that target and aid the host immune defense to repel cancer cells or invading pathogens are rapidly emerging. Antibiotic resistance is among the largest threats to human health globally. Staphylococcus aureus (S. aureus) is the most common bacterial infection, and it poses a challenge to the healthcare system due to its significant ability to develop resistance toward current available therapies. In long-term infections, S. aureus further adapt to avoid clearance by the host immune defense. In this study, we discover a new interaction that allows S. aureus to avoid elimination by the immune system, which likely supports its persistence in the host. Moreover, we find that blocking the specific receptor (PD-1) using antibodies significantly relieves the S. aureus-imposed inhibition. Our findings suggest that therapeutically targeting PD-1 is a possible future strategy for treating certain antibiotic-resistant staphylococcal infections.
-
Immunology and Microbiology
In Biomolecules and Biomedicine on 1 February 2023 by He, W., Hao, S., et al.
The risk of hepatitis B virus (HBV) infection is higher in patients with diabetes mellitus, and diabetes mellitus is one of the metabolic complications of HBV infection. However, the cytokine profile of chronic hepatitis B (CHB) patients with type 2 diabetes mellitus (T2DM) is not fully understood. The aim of this study was to investigate the cytokine expression profile in CHB patients with T2DM, and to assess the regulatory function of cytokines to regulatory T cells (Tregs). Forty-four T2DM patients, 39 CHB patients, 17 patients with CHB and T2DM, and 21 control subjects were enrolled. Cytokine levels in the plasma were measured by Luminex multiplex assay. CD4+CD25+CD127dim/- Tregs were detected by flow cytometry. Tregs were purified and stimulated with recombinant human interleukin-15 (IL-15). The regulation of IL-15 on Tregs function was investigated by measuring cell number, IL-10/IL-35 secretion, and mRNA expression of immune checkpoint molecules in a Tregs+PBMC co-culture system. We found that levels of IL-1α, IL-6, and IL-33 were upregulated, while IFN-α, IL-2, IL-7, and IL-15 were downregulated in T2DM and CHB patients. CHB patients with T2DM had even lower plasma IL-7 and IL-15 levels. Tregs percentage was elevated in T2DM and CHB patients. CHB patients with T2DM had increased levels of Tregs, which correlated negatively with IL-15. Tregs showed stronger inhibitory activity in CHB patients with T2DM than in controls, T2DM, and CHB patients, which presented as reduction in cellular proliferation and induction of IL-10/IL-35 secretion. IL-15 suppressed Tregs function and inhibited the expression of immune checkpoint molecules in Tregs. The current data suggest that insufficient IL-15 levels and decreased responsiveness of Tregs to IL-15 signaling might contribute to strong immune dysfunction in CHB patients with T2DM.
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Cancer Res Commun on 1 January 2023 by Starr, R., Aguilar, B., et al.
Chimeric antigen receptor (CAR) T cell immunotherapy is emerging as a powerful strategy for cancer therapy; however, an important safety consideration is the potential for off-tumor recognition of normal tissue. This is particularly important as ligand-based CARs are optimized for clinical translation. Our group has developed and clinically translated an IL13(E12Y) ligand-based CAR targeting the cancer antigen IL13Rα2 for treatment of glioblastoma (GBM). There remains limited understanding of how IL13-ligand CAR design impacts the activity and selectivity for the intended tumor-associated target IL13Rα2 versus the more ubiquitous unintended target IL13Rα1. In this study, we functionally compared IL13(E12Y)-CARs incorporating different intracellular signaling domains, including first-generation CD3ζ-containing CARs (IL13ζ), second-generation 4-1BB (CD137)-containing or CD28-containing CARs (IL13-BBζ or IL13-28ζ), and third-generation CARs containing both 4-1BB and CD28 (IL13-28BBζ). In vitro coculture assays at high tumor burden establish that second-generation IL13-BBζ or IL13-28ζ outperform first-generation IL13ζ and third-generation IL13-28BBζ CAR designs, with IL13-BBζ providing superior CAR proliferation and in vivo antitumor potency in human xenograft mouse models. IL13-28ζ displayed a lower threshold for antigen recognition, resulting in higher off-target IL13Rα1 reactivity both in vitro and in vivo. Syngeneic mouse models of GBM also demonstrate safety and antitumor potency of murine IL13-BBζ CAR T cells delivered systemically after lymphodepletion. These findings support the use of IL13-BBζ CARs for greater selective recognition of IL13Rα2 over IL13Rα1, higher proliferative potential, and superior antitumor responsiveness. This study exemplifies the potential of modulating factors outside the antigen targeting domain of a CAR to improve selective tumor recognition.
This study reveals how modulating CAR design outside the antigen targeting domain improves selective tumor recognition. Specifically, this work shows improved specificity, persistence, and efficacy of 4-1BB-based IL13-ligand CARs. Human clinical trials evaluating IL13-41BB-CAR T cells are ongoing, supporting the clinical significance of these findings.
© 2023 The Authors; Published by the American Association for Cancer Research.
-
FC/FACS
-
Immunology and Microbiology
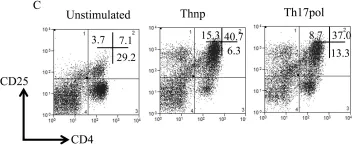
In PLoS One on 4 July 2017 by Bughani, U., Saha, A., et al.
Fig.1.C

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 1