Invasive fungal infections (IFIs) are responsible for elevated rates of morbidity and mortality, causing around of 1.5 million deaths annually worldwide. One of the main causative agents of IFIs is Candida albicans, and non-albicans Candida species have emerged as a spreading global public health concernment. Furthermore, COVID-19 has contributed to a boost in the incidence of IFIs, such as mucormycosis, in which Rhizopus oryzae is the most prevalent causative agent. The effector host immune response against IFIs depends on the activity of T cells, which are susceptible to the regulatory effects triggered by fungal virulence factors. The fungal cell wall plays a crucial role as a virulence factor, and its remodeling compromises the development of a specific T-cell response. The redirection of Jurkat T cells to target Candida spp. by recognizing targets expressed on the fungal cell wall can be facilitated using chimeric antigen receptor (CAR) technology. This study generated an M-CAR that contains an scFv with specificity to α-1,6 mannose backbone of fungal mannan, and the expression of M-CAR on the surface of modified Jurkat cells triggered a strong activation against Candida albicans (hyphae form), Candida tropicalis (hyphae form), Candida parapsilosis (pseudohyphal form), and Candida glabrata (yeast form). Moreover, M-CAR Jurkat cells recognized Rhizopus oryzae spores, which induced high expression of cell activation markers. Thus, a novel Mannan-specific CAR enabled strong signal transduction in modified Jurkat cells in the presence of Candida spp. or R. oryzae.
© 2025 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Product Citations: 209
In Bioengineered on 1 December 2025 by Guimarães, J. G., de Campos, G. Y., et al.
-
Immunology and Microbiology
In Scientific Reports on 11 July 2025 by Li, D., Ma, X., et al.
Parkinson's disease (PD) patients frequently exhibit vitamin D deficiency and an imbalance in T helper 17 (Th17) and regulatory T (Treg) cells, which may contribute to disease pathogenesis. Preclinical evidence suggests vitamin D regulates Th17/Treg balance, but the therapeutic effects of supplementation in PD remain unestablished. This randomized controlled trial investigated peripheral blood levels of vitamin D, Treg, and Th17 cells in PD patients, examined their associations with clinical outcomes, and assessed vitamin D3 supplementation's effects on immunological and motor functions. In this randomized, double-blind, placebo-controlled trial, 51 PD patients and 50 healthy controls (HCs) were enrolled. Thirty PD patients with vitamin D deficiency were randomized to receive vitamin D3 (n = 15) or placebo (vegetable oil, n = 15) for three months. Serum 25(OH)D3 levels were measured by electrochemiluminescence, and Th17/Treg cells were analyzed by flow cytometry. Motor and non-motor symptoms were assessed using standardized scales. Vitamin D3 supplementation significantly increased 25(OH)D3 levels (p < 0.05), reduced Th17 cells (4.62 ± 1.09 to 3.25 ± 1.14, p = 0.003), and elevated Tregs (3.25 ± 0.90 to 4.52 ± 0.95, p = 0.003). Motor function (UPDRS and UPDRS-III) improved in the vitamin D3 group (p < 0.001), while no changes were observed in the placebo group. This preliminary study suggests that vitamin D3 supplementation may restore Th17/Treg balance and potentially alleviate motor symptoms in vitamin D-deficient PD patients, indicating a possible therapeutic strategy.Trial registration: ClinicalTrials.gov: NCT:06539260. Registered 05 August 2024 - Retrospectively registered, https://clinicaltrials.gov/study/NCT06539260 .
© 2025. The Author(s).
-
Immunology and Microbiology
-
Neuroscience
In Scientific Reports on 22 May 2025 by Hou, J., Zhao, J., et al.
Chronic obstructive pulmonary disease (COPD) is a heterogeneous condition, with varying clinical phenotypes and prognoses. Regulatory T cells (Tregs), particularly CD4+FOXP3+ T cell subpopulations, are crucial in modulating immune responses. This study investigates the distribution of two CD4+FOXP3+ T cell subpopulations in bronchoalveolar lavage fluid (BALF) from COPD patients and their association with disease phenotypes and prognosis. Patients were classified into Type A (lower frequencies of inflammatory FOXP3lo T cells) and Type B (higher frequencies of inflammatory FOXP3lo T cells). Type B COPD patients, who demonstrated more severe emphysema, heightened inflammatory responses, faster lung function decline, and more pronounced osteoporosis, showed a significant increase in FOXP3lo non-suppressive T cells. In contrast, Type A patients exhibited a higher proportion of FOXP3hi Treg cells, which correlated with milder disease phenotypes. The distinct distribution of CD4+FOXP3+ T cell subpopulations provides insights into the progression of COPD and suggests that these cells could serve as potential biomarkers for disease severity and prognosis. Further research may offer new therapeutic avenues by targeting these Treg subpopulations in COPD management.
© 2025. The Author(s).
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Drug Design, Development and Therapy on 3 March 2025 by Kong, X., Lin, Y., et al.
Human interleukin-2 (IL-2) stimulates the differentiation and expansion of diverse immune cells dose-dependently. As an immunotherapy agent to treat metastatic cancers, IL-2 has been used in clinical practice and has demonstrated clear antitumor effects; however, its short half-life, the risk of capillary leak syndrome, and the unintended activation of immunosuppressive Treg cells hinder its clinical application. To address these challenges, a novel PEGylated interleukin-2 analogue, SHR-1916, was designed. Its cellular selectivity, efficacy, and improved pharmacokinetic profiles were investigated.
The binding affinities were characterized by surface plasmon resonance (SPR) in vitro. Subsequently, the stimulatory properties were investigated in a murine cell line (CTLL-2), a human cell line (M07e), and human peripheral blood mononuclear cells (PBMCs). To assess the anti-tumor efficacy, a CT-26 colon carcinoma syngeneic model in BALB/c mice and a A375 human melanoma xenograft model using PBMC humanized NCG mice were used in vivo. Moreover, the pharmacokinetic behavior following a single intravenous or subcutaneous dose was evaluated in Sprague-Dawley rats.
SHR-1916 abolished binding to its receptor IL-2Rα, as evidenced by SPR assays, and exerted its activity mainly through binding to IL-2Rβγ, as confirmed by CTLL-2 and M07e cell proliferation assays. In contrast to IL-2, SHR-1916 exhibited a more biased activation of CD8+ T and NK cells compared to Treg cells and stimulated an increase in IFNγ secretion in PBMCs dose-dependently without triggering the release of other potential side effect-associated cytokines. In CT26 colon carcinoma and A375 melanoma models, SHR-1916 significantly reduced the tumor burden. Pharmacokinetic results showed that SHR-1916 had a significantly prolonged half-life in rats.
SHR-1916 exhibited excellent cellular selectivity, anti-tumor efficacies, and improved pharmacokinetics. It has the potential to serve as a novel immunotherapeutic agent designed to enhance IL-2's immune-stimulating activities and promote its tolerability while reducing the immunoregulatory function of Treg cells.
© 2025 Kong et al.
-
Cancer Research
-
Immunology and Microbiology
Single cell suppression profiling of human regulatory T cells.
In Nature Communications on 3 February 2025 by Søndergaard, J. N., Tulyeu, J., et al.
Regulatory T cells (Treg) play an important role in regulating immune homeostasis in health and disease. Traditionally their suppressive function has been assayed by mixing purified cell populations, which does not provide an accurate picture of a physiologically relevant response. To overcome this limitation, we here develop 'single cell suppression profiling of human Tregs' (scSPOT). scSPOT uses a 52-marker CyTOF panel, a cell division detection algorithm, and a whole PBMC system to assess the effect of Tregs on all other cell types simultaneously. In this head-to-head comparison, we find Tregs having the clearest suppressive effects on effector memory CD8 T cells through partial division arrest, cell cycle inhibition, and effector molecule downregulation. Additionally, scSPOT identifies a Treg phenotypic split previously observed in viral infection and propose modes of action by the FDA-approved drugs Ipilimumab and Tazemetostat. scSPOT is thus scalable, robust, widely applicable, and may be used to better understand Treg immunobiology and screen for therapeutic compounds.
© 2025. The Author(s).
-
Immunology and Microbiology
In PLoS One on 25 February 2011 by Nausch, N., Midzi, N., et al.
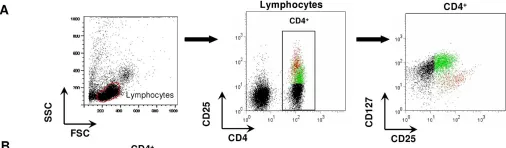
Fig.2.A

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3
In PLoS One on 25 February 2011 by Nausch, N., Midzi, N., et al.
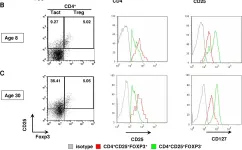
Fig.2.B

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3
In PLoS One on 15 December 2010 by Jana, S., Campbell, H., et al.
Fig.4.A

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3