Hepatocellular carcinoma (HCC) resists immunotherapy due to its immunosuppressive microenvironment. Sarcoma homology 2 domain-containing protein tyrosine phosphatase-1 (SHP-1) inhibits T cell receptor signaling, and its pharmacological inhibition is limited by poor selectivity and membrane permeability. Here, we generated CRISPR-edited SHP-1-knockout (KO) CD8+ T cells to enhance adoptive therapy against HCC. Single-cell RNA sequencing of HCC patient T cells revealed elevated SHP-1 in exhausted subsets. SHP-1-KO T cells exhibited increased effector memory T cells (TEM) proportions and enhanced IFN-γ/Granzyme B/perforin secretion, improving cytotoxicity against HCC lines. In humanized PDX models, SHP-1-KO T cells demonstrated superior tumor-killing activity. Transcriptomics identified upregulated lipid metabolism pathways, with HMGCR as a hub gene. Combining SHP-1-KO T cells with simvastatin (HMGCR inhibitor) synergistically amplified anti-HCC efficacy. This study proposes a dual strategy combining SHP-1-targeted cell therapy and metabolic modulation to overcome immunotherapy resistance, offering a translatable approach for HCC treatment.
© 2025 The Author(s).
Product Citations: 29
In IScience on 18 April 2025 by Liu, H., Ge, W., et al.
-
Cancer Research
Evaluation of the Abdala Vaccine: Antibody and Cellular Response to the RBD Domain of SARS-CoV-2.
In Vaccines on 30 November 2023 by Islas-Vazquez, L., Alvarado-Alvarado, Y. C., et al.
Abdala is a recently released RBD protein subunit vaccine against SARS-CoV-2. A few countries, including Mexico, have adopted Abdala as a booster dose in their COVID-19 vaccination schemes. Despite that, most of the Mexican population has received full-scheme vaccination with platforms other than Abdala; little is known regarding Abdala's immunological features, such as its antibody production and T- and B-cell-specific response induction. This work aimed to study antibody production and the adaptive cellular response in the Mexican population that received the Abdala vaccine as a booster. We recruited 25 volunteers and evaluated their RBD-specific antibody production, T- and B-cell-activating profiles, and cytokine production. Our results showed that the Abdala vaccine increases the concentration of RBD IgG-specific antibodies. Regarding the cellular response, after challenging peripheral blood cultures with RBD, the plasmablast (CD19+CD27+CD38High) and transitional B-cell (CD19+CD21+CD38High) percentages increased significantly, while T cells showed an increased activated phenotype (CD3+CD4+CD25+CD69+ and CD3+CD4+CD25+HLA-DR+). Also, IL-2 and IFN-γ increased significantly in the supernatant of the RBD-stimulated cells. Our results suggest that Abdala vaccination, used as a booster, evokes antibody production and the activation of previously generated memory against the SARS-CoV-2 RBD domain.
-
FC/FACS
-
COVID-19
-
Immunology and Microbiology
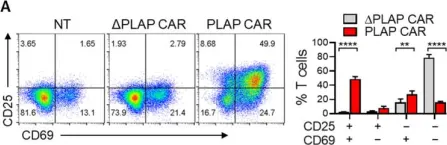
Generation and Functional Characterization of PLAP CAR-T Cells against Cervical Cancer Cells.
In Biomolecules on 14 September 2022 by Yekehfallah, V., Pahlavanneshan, S., et al.
Chimeric antigen receptor (CAR) T-cell therapy is one of the cancer treatment modalities that has recently shown promising results in treating hematopoietic malignancies. However, one of the obstacles that need to be addressed in solid tumors is the on-target and off-tumor cytotoxicity due to the lack of specific tumor antigens with low expression in healthy cells. Placental alkaline phosphatase (PLAP) is a shared placenta- and tumor-associated antigen (TAA) that is expressed in ovarian, cervical, colorectal, and prostate cancers and is negligible in normal cells. In this study, we constructed second-generation CAR T cells with a fully human scFv against PLAP antigen andthen evaluated the characteristics of PLAP CAR T cells in terms of tonic signaling and differentiation in comparison with ΔPLAP CAR T cells and CD19 CAR T cells. In addition, by co-culturing PLAP CAR T cells with HeLa and CaSki cells, we analyzed the tumor-killing functions and the secretion of anti-tumor molecules. Results showed that PLAP CAR T cells not only proliferated during co-culture with cancer cells but also eliminated them in vitro. We also observed increased secretion of IL-2, granzyme A, and IFN-γ by PLAP CAR T cells upon exposure to the target cells. In conclusion, PLAP CAR T cells are potential candidates for further investigation in cervical cancer and, potentially, other solid tumors.
-
FC/FACS
-
Homo sapiens (Human)
-
Biochemistry and Molecular biology
-
Cancer Research
-
Immunology and Microbiology
In IScience on 21 January 2022 by Law, H., Mach, M., et al.
T follicular helper (Tfh) cells provide critical help to B cells during the germinal center (GC) reaction to facilitate generation of protective humoral immunity. Accessing the human lymph node (LN) to study the commitment of CD4 T cells to GC Tfh cell differentiation during in vivo vaccine responses is difficult. We used ultrasound guided fine needle biopsy to monitor recall responses in axillary LNs to seasonal influenza vaccination in healthy volunteers. Specific expansion of GC cell subsets occurred exclusively within draining LNs five days postvaccination. Draining LN GC Tfh and precursor-Tfh cells express higher levels of CD38, ICOS, and Ki67, indicating they were significantly more activated, motile, and proliferating, compared to contralateral LN cells. These observations provide insight into the early expansion phase of the human Tfh lineage within LNs during a vaccine induced memory response and highlights early LN immune responses may not be reflected in the periphery.
© 2021 The Author(s).
-
Immunology and Microbiology
Determinants of anti-PD-1 response and resistance in clear cell renal cell carcinoma.
In Cancer Cell on 8 November 2021 by Au, L., Hatipoglu, E., et al.
ADAPTeR is a prospective, phase II study of nivolumab (anti-PD-1) in 15 treatment-naive patients (115 multiregion tumor samples) with metastatic clear cell renal cell carcinoma (ccRCC) aiming to understand the mechanism underpinning therapeutic response. Genomic analyses show no correlation between tumor molecular features and response, whereas ccRCC-specific human endogenous retrovirus expression indirectly correlates with clinical response. T cell receptor (TCR) analysis reveals a significantly higher number of expanded TCR clones pre-treatment in responders suggesting pre-existing immunity. Maintenance of highly similar clusters of TCRs post-treatment predict response, suggesting ongoing antigen engagement and survival of families of T cells likely recognizing the same antigens. In responders, nivolumab-bound CD8+ T cells are expanded and express GZMK/B. Our data suggest nivolumab drives both maintenance and replacement of previously expanded T cell clones, but only maintenance correlates with response. We hypothesize that maintenance and boosting of a pre-existing response is a key element of anti-PD-1 mode of action.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
-
Cancer Research
In Biomolecules on 14 September 2022 by Yekehfallah, V., Pahlavanneshan, S., et al.
Fig.3.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Biomolecules by CiteAb, provided under a CC-BY license
Image 1 of 1