Product Citations: 124
Optimal Timing of PD-1/PD-L1 Blockade Protects Organ Function During Sepsis.
In Inflammation on 23 August 2024 by Xiang, J., Wang, Y., et al.
In Advanced Biology on 1 July 2024 by Huang, Y., Zhong, M., et al.
Multiple myeloma (MM) stands as a prevalent hematological malignancy, primarily incurable, originating from plasma cell clones. MM's progression encompasses genetic abnormalities and disruptions in the bone marrow microenvironment, leading to tumor proliferation, immune dysfunction, and compromised treatment outcomes. Emerging evidence highlights the critical role of regulatory T cells (Tregs) in MM progression, suggesting that targeting Tregs could enhance immune functionality and treatment efficacy. In this study, a notable increase in Treg proportions within MM patients' bone marrow (BM) compared to healthy individuals is observed. Additionally, it is found that the bromodomain and extraterminal domain (BET) inhibitor JQ1 selectively diminishes Treg percentages in MM patients' BM and reduces TGF-β1-induced Tregs. This reduction occurs via inhibiting cell viability and promoting apoptosis. RNA sequencing further indicates that JQ1's inhibitory impact on Tregs likely involves upregulating STAT3 and suppressing PD-1 expression. Collectively, these findings suggest JQ1's potential to modulate Tregs, bolstering the immune response in MM and introducing a promising avenue for MM immunotherapy.
© 2024 Wiley‐VCH GmbH.
In NPJ Systems Biology and Applications on 29 May 2024 by Magni, S., Sawlekar, R., et al.
The discovery of upstream regulatory genes of a gene of interest still remains challenging. Here we applied a scalable computational method to unbiasedly predict candidate regulatory genes of critical transcription factors by searching the whole genome. We illustrated our approach with a case study on the master regulator FOXP3 of human primary regulatory T cells (Tregs). While target genes of FOXP3 have been identified, its upstream regulatory machinery still remains elusive. Our methodology selected five top-ranked candidates that were tested via proof-of-concept experiments. Following knockdown, three out of five candidates showed significant effects on the mRNA expression of FOXP3 across multiple donors. This provides insights into the regulatory mechanisms modulating FOXP3 transcriptional expression in Tregs. Overall, at the genome level this represents a high level of accuracy in predicting upstream regulatory genes of key genes of interest.
© 2024. The Author(s).
-
Immunology and Microbiology
In Cell Reports Medicine on 16 April 2024 by Albanese, M., Chen, H. R., et al.
Immune cell phenotyping frequently detects lineage-unrelated receptors. Here, we report that surface receptors can be transferred from primary macrophages to CD4 T cells and identify the Fcγ receptor CD32 as driver and cargo of this trogocytotic transfer. Filamentous CD32+ nanoprotrusions deposit distinct plasma membrane patches onto target T cells. Transferred receptors confer cell migration and adhesion properties, and macrophage-derived membrane patches render resting CD4 T cells susceptible to infection by serving as hotspots for HIV-1 binding. Antibodies that recognize T cell epitopes enhance CD32-mediated trogocytosis. Such autoreactive anti-HIV-1 envelope antibodies can be found in the blood of HIV-1 patients and, consistently, the percentage of CD32+ CD4 T cells is increased in their blood. This CD32-mediated, antigen-independent cell communication mode transiently expands the receptor repertoire and functionality of immune cells. HIV-1 hijacks this mechanism by triggering the generation of trogocytosis-promoting autoantibodies to gain access to immune cells critical to its persistence.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
De novoPADI4-mediated citrullination of histone H3 stimulates HIV-1 transcription
Preprint on BioRxiv : the Preprint Server for Biology on 18 March 2024 by Love, L., Jütte, B. B., et al.
HIV-1 infection establishes a reservoir of long-lived cells with integrated proviral DNA that can persist despite antiretroviral therapy (ART). The mechanisms governing the transcriptional regulation of the provirus are complex and incompletely understood. Here, we investigated the role of histone H3 citrullination, a post-translational modification catalyzed by protein-arginine deiminase type-4 (PADI4), in HIV-1 transcription and latency. We found that PADI4 inhibition by GSK484 reduced HIV-1 transcription after T cell activation in ex vivo cultures of CD4 T cells from viremic and ART treated people living with HIV-1 (PLWH). The effect was more pronounced in the viremic group. Using cell models of HIV-1 latency, we showed that PADI4-mediated citrullination of histone H3 occurred at the HIV-1 promoter upon T cell stimulation which facilitated proviral transcription. Citrullination of the H3R8 residue prevented heterochromatin formation. We also demonstrated that HIV-1 preferentially integrated into genomic regions marked by H3 citrullination and that proviruses in H3 citrullinated chromatin were more transcriptionally active and less prone to latency than those in non-citrullinated chromatin. Our data reveal a novel mechanism of HIV-1 transcriptional regulation by PADI4 and H3 citrullination and suggest a potential therapeutic target for reducing the size of the latent reservoir, as an approach to cure. Highlights The PADI4 enzyme stimulates HIV-1 transcription during T cell activation. PADI4 citrullinates histone H3 at the HIV-1 promoter upon T cell activation and inhibiting PADI4 reduces HIV-1 reactivation in ex vivo CD4 T cells from people living with HIV-1. H3cit is mostly found at gene promoters, and productive HIV-1 proviruses are more likely than latent or reactivatable proviruses, to integrate in chromatin susceptible for citrullination. H3cit prevents latency establishment by interfering with the binding of HP1α to H3K9me3.
-
Biochemistry and Molecular biology
-
Genetics
In Cells on 1 January 2021 by Fiori, A., Uhlig, S., et al.
Fig.4.B

-
FC/FACS
-
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 2
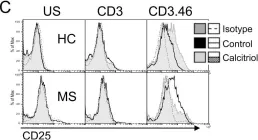
In PLoS One on 13 November 2012 by Kickler, K., Ni Choileain, S., et al.
Fig.3.C

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 2