Exosomes, nanoscale vesicles with high biocompatibility, were engineered to express human epidermal growth factor receptor 2 (HER2)-binding peptides and carry miR-34a, targeting HER2 and programmed death-ligand 1 (PD-L1)-positive breast cancer cells.
An in vivo xenograft breast cancer model was established by subcutaneously injecting breast cancer cells of both HER2 and PD-L1 positivity (SK-BR3 cells) into the buttocks of BALB/c nude mice. miR-34a-loaded HER2-targeting exosomes, termed tEx[34a], were engineered by transfecting human adipose-derived mesenchymal stem cells with the pDisplay vector to express HER2-binding peptides (P51 peptide). Purified exosomes were then loaded with miR-34a, a tumor-suppressor miRNA, using the Exo-Fect transfection kit, creating tEx[34a] for targeted cancer therapy.
Intravenous administration of miR-34a-loaded HER2-targeting exosomes, referred to as tEx[34a], demonstrated superior targetability compared to other materials, such as natural exosomes, miR-34a-loaded exosomes, and unloaded HER2-targeting exosomes. In vivo experiments using mouse breast cancer xenograft models revealed that the administration of tEx[34a] resulted in the smallest tumor size and lowest tumor weight when compared to all other groups. Notably, tEx[34a] treatment significantly reduced PD-L1 expression in breast cancer tissue compared to the other groups. Furthermore, tEx[34a] administration led to the highest upregulation of pro-apoptotic markers (Bax, PARP, and BIM) and the lowest downregulation of the anti-apoptotic marker Bcl-xL, as confirmed through various methods including RT-PCR, Western blot analysis, and immunofluorescence.
MiR-34a-loaded HER2-targeting exosomes demonstrate strong anticancer efficacy by selectively binding to HER2-positive breast cancer cells and effectively suppressing PD-L1 expression.
© The Authors 2025.
Product Citations: 154
In Journal of Breast Cancer on 1 June 2025 by Sun, W. Y., Lee, D. S., et al.
-
Cancer Research
In STAR Protocols on 21 March 2025 by Funasaki, S., Miyamura, Y., et al.
Angiogenesis begins as endothelial cells migrate, forming a sprouting tip and subsequent growth-rich stalk cells. Here, we present a protocol for transcriptomic and epigenomic analyses of tip-like cells in cultured endothelial cells. We describe steps for stimulating human umbilical vein endothelial cells (HUVECs) with vascular endothelial cell growth factor (VEGF) to generate tip-like cells. We then detail procedures for library preparation for single-cell RNA sequencing (RNA-seq) and chromatin immunoprecipitation sequencing (ChIP-seq), and data analysis. This scalable protocol is also applicable to diverse omics studies, including proteomics and metabolomics. For complete details on the use and execution of this protocol, please refer to Miyamura et al.1.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Identification of human cranio-maxillofacial skeletal stem cells for mandibular development.
In Science Advances on 3 January 2025 by Wang, Z., Wang, K., et al.
Compared with long bone that arises from the mesoderm, the major portion of the maxillofacial bones and the front bone of the skull are derived from cranial neural crest cells and undergo intramembranous ossification. Human skeletal stem cells have been identified in embryonic and fetal long bones. Here, we describe a single-cell atlas of the human embryonic mandible and identify a population of cranio-maxillofacial skeletal stem cells (CMSSCs). These CMSSCs are marked by interferon-induced transmembrane protein 5 (IFITM5) and are specifically located around the periosteum of the jawbone and frontal bone. Additionally, these CMSSCs exhibit strong self-renewal and osteogenic differentiation capacities but lower chondrogenic differentiation potency, mediating intramembranous bone formation without cartilage formation. IFITM5+ cells are also observed in the adult jawbone and exhibit functions similar to those of embryonic CMSSCs. Thus, this study identifies CMSSCs that orchestrate the intramembranous ossification of cranio-maxillofacial bones, providing a deeper understanding of cranio-maxillofacial skeletal development and promising seed cells for bone repair.
-
Stem Cells and Developmental Biology
An organotypic atlas of human vascular cells.
In Nature Medicine on 1 December 2024 by Barnett, S. N., Cujba, A. M., et al.
The human vascular system, comprising endothelial cells (ECs) and mural cells, covers a vast surface area in the body, providing a critical interface between blood and tissue environments. Functional differences exist across specific vascular beds, but their molecular determinants across tissues remain largely unknown. In this study, we integrated single-cell transcriptomics data from 19 human organs and tissues and defined 42 vascular cell states from approximately 67,000 cells (62 donors), including angiotypic transitional signatures along the arterial endothelial axis from large to small caliber vessels. We also characterized organotypic populations, including splenic littoral and blood-brain barrier ECs, thus clarifying the molecular profiles of these important cell states. Interrogating endothelial-mural cell molecular crosstalk revealed angiotypic and organotypic communication pathways related to Notch, Wnt, retinoic acid, prostaglandin and cell adhesion signaling. Transcription factor network analysis revealed differential regulation of downstream target genes in tissue-specific modules, such as those of FOXF1 across multiple lung vascular subpopulations. Additionally, we make mechanistic inferences of vascular drug targets within different vascular beds. This open-access resource enhances our understanding of angiodiversity and organotypic molecular signatures in human vascular cells, and has therapeutic implications for vascular diseases across tissues.
© 2024. The Author(s).
In STAR Protocols on 20 September 2024 by Remmert, C., Otgonbayar, M., et al.
Here, we present a protocol for producing a microfluidic vessel-on-chip platform using human pluripotent stem cell-derived endothelial cells (SC-ECs). We describe steps for manufacturing the 3D-printed chip, cell culturing to generate SC-ECs, hydrogel patterning, and the formation and cultivation of barrier-forming vessels. We then detail procedures for the retrieval of cells and media from the open microfluidic chip platform to enable multi-omics analysis. For complete details on the use and execution of this protocol, please refer to Marder et al.1.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Stem Cells and Developmental Biology
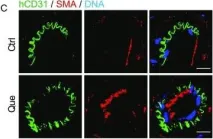
In Protein Cell on 1 June 2019 by Geng, L., Liu, Z., et al.
Fig.4.C

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Protein Cell by CiteAb, provided under a CC-BY license
Image 1 of 1