Knee arthrofibrosis, characterized by excessive matrix protein production and deposition, substantially impairs basic daily functions, causing considerable distress and financial burden. However, the underlying pathomechanisms remain unclear. Here, we characterized the heterogeneous cell populations and cellular pathways by combination of flow cytometry and single-cell RNA-seq analysis of synovial tissues from six patients with or without knee arthrofibrosis. Increased macrophages and fibroblasts were observed with decreased numbers of fibroblast-like synoviocytes, endothelial cells, vascular smooth muscle cells, and T cells in the arthrofibrosis group compared with negative controls. Notably, fibroblasts were discovered to interact with macrophages, and lead to fibrosis through TGF-β pathway induced CCN2 expression in fibroblasts. CCN2 was demonstrated to be required for fibroblast pro-fibrotic functions (activation, proliferation, and migration) through TGFBR/SMAD pathway. The expression of CCN2 was positively correlated with the collagen volume and TGF-β expression and negatively associated with patient-reported outcome measures in another cohort of patients with knee arthrofibrosis. Our study reveals the role of CCN2 in the fibroblast-macrophage interaction through TGF-β pathway which might help to shed light on CCN2 as a potential biomarker.
© 2025. The Author(s).
Product Citations: 113
In Bone Research on 24 February 2025 by Li, Z., Jiang, J., et al.
-
FC/FACS
-
Homo sapiens (Human)
-
Genetics
-
Immunology and Microbiology
In Life (Basel, Switzerland) on 28 January 2025 by Saino, O., Ogawa, Y., et al.
The efficacy of hematopoietic stem cell (HSC) therapy for cerebral infarction has been previously demonstrated. However, the lack of response in some patients has hindered its widespread use. To establish HSC therapy as a standard treatment, it is important to examine the causes of non-responsiveness. In this study, we aimed to identify the specifications of transplanted cells based on their therapeutic mechanisms to predict treatment success. We found that HSC therapy activates injured cerebral endothelial cells via gap junctions because cell adhesion between HSCs and the endothelium plays an essential role in cellular communication via gap junctions. The expression of the adhesion molecule integrin β2 (CD18) in CD34-positive (CD34+) cells was identified as critical for the therapeutic effect on cerebral infarction in a murine model. Cells with low CD18 expression exhibited a weaker therapeutic effect than cells with high CD18 expression, even when the same number of HSCs was administered. The expression of CD18 in CD34+ cells can be used as a specification marker for transplanted HSCs and is useful for identifying non-responders. Furthermore, quantification of CD18 expression is crucial for evaluating the cellular potential of cell-based therapies for diseases where therapeutic effects are mediated through cell adhesion.
-
Stem Cells and Developmental Biology
In Nature Communications on 27 November 2024 by Lim, A. A., Pouyabahar, D., et al.
The sinoatrial node regulates the heart rate throughout life. Failure of this primary pacemaker results in life-threatening, slow heart rhythm. Despite its critical function, the cellular and molecular composition of the human sinoatrial node is not resolved. Particularly, no cell surface marker to identify and isolate sinoatrial node pacemaker cells has been reported. Here we use single-nuclei/cell RNA sequencing of fetal and human pluripotent stem cell-derived sinoatrial node cells to reveal that they consist of three subtypes of pacemaker cells: Core Pacemaker, Sinus Venosus, and Transitional Cells. Our study identifies a host of sinoatrial node pacemaker markers including MYH11, BMP4, and the cell surface antigen CD34. We demonstrate that sorting for CD34+ cells from stem cell differentiation cultures enriches for sinoatrial node cells exhibiting a functional pacemaker phenotype. This sinoatrial node pacemaker cell surface marker is highly valuable for stem cell-based disease modeling, drug discovery, cell replacement therapies, and the targeted delivery of therapeutics to sinoatrial node cells in vivo using antibody-drug conjugates.
© 2024. The Author(s).
-
FC/FACS
Preprint on BioRxiv : the Preprint Server for Biology on 7 September 2024 by Lim, A. A., Pouyabahar, D., et al.
The sinoatrial node (SAN) regulates the heart rate throughout life. Failure of this primary pacemaker results in life-threatening, slow heart rhythm. Despite its important function, the cellular and molecular composition of the human SAN is not completely resolved. Particularly, no cell surface marker to identify and isolate SAN pacemaker cells has been reported to date. Here we used single-nuclei/cell RNA sequencing of fetal and human pluripotent stem cell (hPSC)- derived SAN cells and show that the SAN consists of three subtypes of pacemaker cells, including Core SAN, SAN, and Transitional Cells. Our study identified a host of novel Core SAN markers including MYH11, BMP4, and the cell surface antigen CD34. We demonstrate that sorting for CD34 + cells from cardiac hPSC differentiations enriches for SAN cells with a functional pacemaker phenotype. This novel SAN pacemaker cell surface marker is highly valuable for future hPSC- based disease modelling, drug discovery, cell replacement therapies, as well as the delivery of therapeutics to SAN cells in vivo using antibody-drug conjugates.
Protocol for differentiation of functional macrophages from human induced pluripotent stem cells.
In STAR Protocols on 15 March 2024 by Jeong, S., Chang, H., et al.
Human induced pluripotent stem cell (hiPSC)-derived macrophages provide a valuable tool for disease modeling and drug discovery. Here, we present a protocol to generate functional macrophages from hiPSCs using a feeder-free hematopoietic differentiation technique. We describe steps for preparing hiPSCs, mesodermal differentiation, hematopoietic commitment, and macrophage differentiation and expansion. We then detail assays to characterize their phenotype, polarization, and phagocytic functions. The functional macrophages generated here could be used to generate organoids for disease modeling and drug discovery studies. For complete details on the use and execution of this protocol, please refer to Jeong et al.1 and Heo et al.2.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
Stem Cells and Developmental Biology
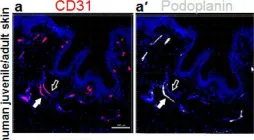
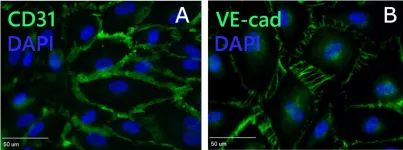
In Cells on 21 March 2022 by Ruetsche, D., Michalak-Mićka, K., et al.
Fig.1.A

-
IHC-IF
-
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 5
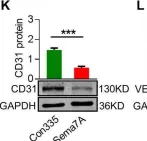
In Cell Death Dis on 10 August 2020 by Hong, L., Li, F., et al.
Fig.1.K

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 5
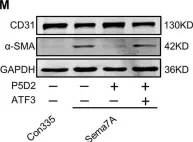
In Cell Death Dis on 10 August 2020 by Hong, L., Li, F., et al.
Fig.4.M

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 5
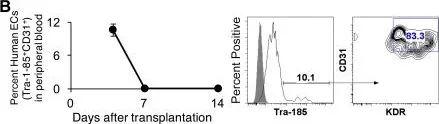
In Stem Cells Transl Med on 1 March 2017 by Gori, J. L., Butler, J. M., et al.
Fig.2.B

-
FC/FACS
-
Macaca mulatta (Rhesus Monkey)
Collected and cropped from Stem Cells Transl Med by CiteAb, provided under a CC-BY license
Image 1 of 5
In PLoS One on 16 June 2015 by Tasev, D., van Wijhe, M. H., et al.
Fig.2.A

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 5