The current understanding of humoral immune response in cancer patients suggests that tumors may be infiltrated with diffuse B cells of extra-tumoral origin or may develop organized lymphoid structures, where somatic hypermutation and antigen-driven selection occur locally. These processes are believed to be significantly influenced by the tumor microenvironment through secretory factors and biased cell-cell interactions. To explore the manifestation of this influence, we used deep unbiased immunoglobulin profiling and systematically characterized the relationships between B cells in circulation, draining lymph nodes (draining LNs), and tumors in 14 patients with three human cancers. We demonstrated that draining LNs are differentially involved in the interaction with the tumor site, and that significant heterogeneity exists even between different parts of a single lymph node (LN). Next, we confirmed and elaborated upon previous observations regarding intratumoral immunoglobulin heterogeneity. We identified B cell receptor (BCR) clonotypes that were expanded in tumors relative to draining LNs and blood and observed that these tumor-expanded clonotypes were less hypermutated than non-expanded (ubiquitous) clonotypes. Furthermore, we observed a shift in the properties of complementarity-determining region 3 of the BCR heavy chain (CDR-H3) towards less mature and less specific BCR repertoire in tumor-infiltrating B-cells compared to circulating B-cells, which may indicate less stringent control for antibody-producing B cell development in tumor microenvironment (TME). In addition, we found repertoire-level evidence that B-cells may be selected according to their CDR-H3 physicochemical properties before they activate somatic hypermutation (SHM). Altogether, our work outlines a broad picture of the differences in the tumor BCR repertoire relative to non-tumor tissues and points to the unexpected features of the SHM process.
© 2024, Krasik, Bryushkova et al.
Product Citations: 89
Systematic evaluation of intratumoral and peripheral BCR repertoires in three cancers.
In eLife on 20 January 2025 by Krasik, S. V., Bryushkova, E. A., et al.
-
Homo sapiens (Human)
In Frontiers in Immunology on 27 December 2024 by Yang, C., You, J., et al.
The use of programmed death-1 (PD-1) inhibitors in the neoadjuvant setting for patients with resectable stage III NSCLC has revolutionized this field in recent years. However, there is still 40%-60% of patients do not benefit from this approach. The complex interactions between immune cell subtypes and tertiary lymphoid structures (TLSs) within the tumor microenvironment (TME) may influence prognosis and the response to immunochemotherapy. This study aims to assess the relationship between immune cells subtypes and TLSs to better understand their impact on immunotherapy response.
This study initially compared the tertiary lymphoid structures (TLSs) density among patients who underwent immunochemotherapy, chemotherapy and upfront surgery using 123 tumor samples from stage-matched patients. Multiplex immunohistochemistry (mIHC) was employed to analyze the spatial distribution of PD-L1+CD11c+ cells and PD1+CD8+ T cells within TLSs. Cytometry by time-of-flight (CyTOF) was used to assess immune cell dynamics in paired biopsy and resection specimens from six patients who underwent immunochemotherapy. Key immune cells were validated in newly collected samples using flow cytometry, mIHC, and in vitro CAR-T cells model.
Patients who underwent neoadjuvant chemotherapy or immunochemotherapy exhibited increased TLSs compared to those who opted for upfront surgery. The TLS area-to-tumor area ratio distinguished pCR+MPR and NR patients in the immunochemotherapy group. Spatial analysis revealed variations in the distance between PD-L1+CD11c+ cells and PD1+CD8+ T cells within TLSs in the immunochemotherapy group. CyTOF analysis revealed an increase in the frequency of key immune cells (CCR7+CD127+CD69+CD4+ and CD38+CD8+ cells) following combined therapy. Treatment responders exhibited an increase in CCR7+CD4+ T cells, whereas CD38+CD8+ T cells were associated with compromised treatment effectiveness.
Immunochemotherapy and chemotherapy increase TLSs and granzyme B+ CD8+ T cells in tumors. The TLS area-to-tumor ratio distinguishes responders from non-responders, with PD-L1+ dendritic cells near CD8+PD-1+ T cells linked to efficacy, suggesting that PD-1 inhibitors disrupt harmful interactions. Post-immunochemotherapy, CD8+ T cells increase, but CD38+CD8+ T cells show reduced functionality. These findings highlight the complex immune dynamics and their implications for NSCLC treatment.
Copyright © 2024 Yang, You, Wang, Chen, Tang, Chen, Zhong, Song, Long, Xiang, Zhao and Xia.
-
Cancer Research
-
Immunology and Microbiology
In Biotechnologia on 23 October 2024 by Baran, J., Kuryk, Ł., et al.
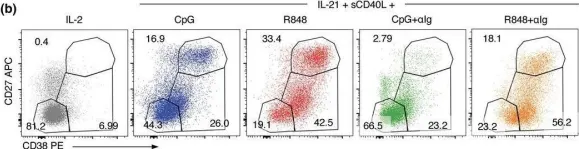
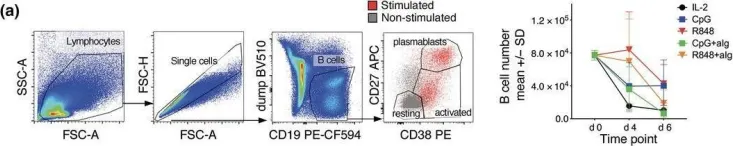
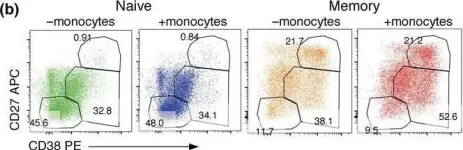
Screening for effective vaccines requires broad studies on their immunogenicity in vitro and ex vivo . We used a PBMC-based system to assess changes in CD4+ T cells, CD8+ T cells, and CD19+ B cells upon stimulation with different combinations of antigens and adjuvants. We studied the activation mechanism using flow cytometry and two different adenoviral adjuvants characterized by the presence or absence of costimulatory ligands for the ICOS and CD40 receptors. Our studies identified the cellular targets and molecular mechanisms driving ongoing switched-antibody diversification. Class-switched memory B cells were the main precursor cells (95.03% ± 0.38 vs. mock 82.33% ± 0.45, P < 0.05) after treatment with the immunogenic formula: adenovirus armed (MIX1) or not (MIX2) with the ICOS and CD40 ligand, the recombinant receptor binding domain (rRBD), and Lentifect™ SARS-CoV-2 spike-pseudotyped lentivirus (GeneCopoeia, USA). Bcell class-switching towards the IgG+IgM+- positive phenotypes was noted (~50-fold increase vs. mock, P < 0.05). A significant increase was observed in the CD8+TEM population of the MIX1 (~2-fold, P < 0.05) and MIX2 (~4.7-fold, P < 0.05) treated samples. CD8+TEMRA increased when PBMCs were treated with MIX2 (9.63% ± 0.90, P < 0.05) vs. mock (2.63% ± 1.96). Class-switched memory B cells were the dominant antigen-specific cells in primary reactions. We indicated a correlation between the protection offered by vaccine regimens and their ability to induce high frequencies of multifunctional T cells.
© 2024 Institute of Bioorganic Chemistry, Polish Academy of Sciences.
-
FC/FACS
-
COVID-19
-
Immunology and Microbiology
CREB1 promotes expression of immune checkpoint HLA-E leading to immune escape in multiple myeloma.
In Leukemia on 1 August 2024 by Ismael, A., Robinette, A. J., et al.
Multiple myeloma (MM) cells effectively escape anti-tumoral immunity to survive in the tumor microenvironment (TME). Herein, we identify non-classical major histocompatibility complex (MHC) class I molecule HLA-E as a major contributing factor in immune escape. Clinically, HLA-E expression correlates with aggressive disease features such as t(4;14) and CD56 expression and is induced by IFN-gamma (IFN-γ) in the TME. We discovered that HLA-E is regulated by cAMP responsive element binding protein 1 (CREB1) transcription factor by direct promoter binding; genomic and pharmacological inhibition of CREB1 reduced HLA-E levels even in the presence of IFN-γ or IFN-γ activating agents, such as immunomodulatory drugs and panobinostat. HLA-E binds to natural killer group 2A (NKG2A), delivering an inhibitor signal to natural killer (NK) cells. Treatment with a CREB1 inhibitor was able to restore NK cell-mediated cytotoxicity against MM cell lines and patient samples. In conclusion, our results strongly demonstrate that CREB1 inhibition promotes anti-tumoral immunity in MM by limiting HLA-E expression and enhancing the activity of NK cells.
© 2024. The Author(s).
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
FLT3L governs the development of partially overlapping hematopoietic lineages in humans and mice.
In Cell on 23 May 2024 by Momenilandi, M., Levy, R., et al.
FMS-related tyrosine kinase 3 ligand (FLT3L), encoded by FLT3LG, is a hematopoietic factor essential for the development of natural killer (NK) cells, B cells, and dendritic cells (DCs) in mice. We describe three humans homozygous for a loss-of-function FLT3LG variant with a history of various recurrent infections, including severe cutaneous warts. The patients' bone marrow (BM) was hypoplastic, with low levels of hematopoietic progenitors, particularly myeloid and B cell precursors. Counts of B cells, monocytes, and DCs were low in the patients' blood, whereas the other blood subsets, including NK cells, were affected only moderately, if at all. The patients had normal counts of Langerhans cells (LCs) and dermal macrophages in the skin but lacked dermal DCs. Thus, FLT3L is required for B cell and DC development in mice and humans. However, unlike its murine counterpart, human FLT3L is required for the development of monocytes but not NK cells.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
FC/FACS
-
Homo sapiens (Human)
In Clin Transl Immunology on 18 December 2019 by Auladell, M., Nguyen, T. H. O., et al.
Fig.1.B

-
FC/FACS
-
Collected and cropped from Clin Transl Immunology by CiteAb, provided under a CC-BY license
Image 1 of 4
In Clin Transl Immunology on 18 December 2019 by Auladell, M., Nguyen, T. H. O., et al.
Fig.1.A

-
FC/FACS
-
Collected and cropped from Clin Transl Immunology by CiteAb, provided under a CC-BY license
Image 1 of 4
In Clin Transl Immunology on 18 December 2019 by Auladell, M., Nguyen, T. H. O., et al.
Fig.5.B

-
FC/FACS
-
Collected and cropped from Clin Transl Immunology by CiteAb, provided under a CC-BY license
Image 1 of 4
In PLoS Pathog on 1 September 2018 by Ahmed, A., Adiga, V., et al.
Fig.4.C

-
FC/FACS
-
Collected and cropped from PLoS Pathog by CiteAb, provided under a CC-BY license
Image 1 of 4