During embryogenesis, human hematopoietic stem cells (HSCs) first emerge in the aorta-gonad-mesonephros (AGM) region via transformation of specialized hemogenic endothelial (HE) cells into premature HSC precursors. This process is termed endothelial-to-hematopoietic transition (EHT), in which the HE cells undergo drastic functional and morphological changes from flat, anchorage-dependent endothelial cells to free-floating round hematopoietic cells. Despite its essential role in human HSC development, molecular mechanisms underlying the EHT are largely unknown. This is due to lack of methods to visualize the emergence of human HSC precursors in real time in contrast to mouse and other model organisms. In this study, by inducing HE from human pluripotent stem cells in feeder-free monolayer cultures, we achieved real-time observation of the human EHT in vitro. By continuous observation and single-cell tracking in the culture, it was possible to visualize a process that a single endothelial cell gives rise to a hematopoietic cell and subsequently form a hematopoietic-cell cluster. The EHT was also confirmed by a drastic HE-to-HSC switching in molecular marker expressions. Notably, HSC precursor emergence was not linked to asymmetric cell division, whereas the hematopoietic cell cluster was formed through proliferation and assembling of the floating cells after the EHT. These results reveal unappreciated dynamics in the human EHT, and we anticipate that our human EHT model in vitro will provide an opportunity to improve our understanding of the human HSC development.
2024 THE BIOPHYSICAL SOCIETY OF JAPAN.
Product Citations: 34
In Biophysics and Physicobiology on 23 August 2024 by Yoneda, Y., Kato, H., et al.
-
Stem Cells and Developmental Biology
Altered erythropoiesis via JAK2 and ASXL1 mutations in myeloproliferative neoplasms.
In Experimental Hematology on 1 April 2024 by Collins, T. B., Laranjeira, A. B. A., et al.
Myeloproliferative neoplasms (MPNs) are driven by hyperactivation of JAK-STAT signaling but can demonstrate skewed hematopoiesis upon acquisition of additional somatic mutations. Here, using primary MPN samples and engineered embryonic stem cells, we demonstrate that mutations in JAK2 induced a significant increase in erythroid colony formation, whereas mutations in additional sex combs-like 1 (ASXL1) led to an erythroid colony defect. RNA-sequencing revealed upregulation of protein arginine methyltransferase 6 (PRMT6) induced by mutant ASXL1. Furthermore, genetic perturbation of PRMT6 exacerbated the MPN disease burden, including leukemic engraftment and splenomegaly, in patient-derived xenograft models, highlighting a novel tumor-suppressive function of PRMT6. However, augmented erythroid potential and bone marrow human CD71+ cells following PRMT6 knockdown were reserved only for primary MPN samples harboring ASXL1 mutations. Last, treatment of CD34+ hematopoietic/stem progenitor cells with the PRMT6 inhibitor EPZ020411 induced expression of genes involved in heme metabolism, hemoglobin, and erythropoiesis. These findings highlight interactions between JAK2 and ASXL1 mutations and a unique erythroid regulatory network in the context of mutant ASXL1.
Copyright © 2024 ISEH -- Society for Hematology and Stem Cells. Published by Elsevier Inc. All rights reserved.
-
Cancer Research
-
Cardiovascular biology
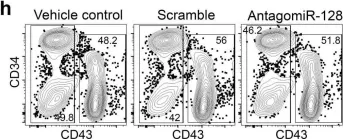
Haematopoietic stem and progenitor cell heterogeneity is inherited from the embryonic endothelium.
In Nature Cell Biology on 1 August 2023 by Ghersi, J. J., Baldissera, G., et al.
Definitive haematopoietic stem and progenitor cells (HSPCs) generate erythroid, lymphoid and myeloid lineages. HSPCs are produced in the embryo via transdifferentiation of haemogenic endothelial cells in the aorta-gonad-mesonephros (AGM). HSPCs in the AGM are heterogeneous in differentiation and proliferative output, but how these intrinsic differences are acquired remains unanswered. Here we discovered that loss of microRNA (miR)-128 in zebrafish leads to an expansion of HSPCs in the AGM with different cell cycle states and a skew towards erythroid and lymphoid progenitors. Manipulating miR-128 in differentiating haemogenic endothelial cells, before their transition to HSPCs, recapitulated the lineage skewing in both zebrafish and human pluripotent stem cells. miR-128 promotes Wnt and Notch signalling in the AGM via post-transcriptional repression of the Wnt inhibitor csnk1a1 and the Notch ligand jag1b. De-repression of cskn1a1 resulted in replicative and erythroid-biased HSPCs, whereas de-repression of jag1b resulted in G2/M and lymphoid-biased HSPCs with long-term consequence on the respective blood lineages. We propose that HSPC heterogeneity arises in the AGM endothelium and is programmed in part by Wnt and Notch signalling.
© 2023. The Author(s).
-
FC/FACS
-
Cell Biology
-
Stem Cells and Developmental Biology
In Nature Cell Biology on 1 May 2022 by Luff, S. A., Creamer, J. P., et al.
The generation of haematopoietic stem cells (HSCs) from human pluripotent stem cells (hPSCs) is a major goal for regenerative medicine. During embryonic development, HSCs derive from haemogenic endothelium (HE) in a NOTCH- and retinoic acid (RA)-dependent manner. Although a WNT-dependent (WNTd) patterning of nascent hPSC mesoderm specifies clonally multipotent intra-embryonic-like HOXA+ definitive HE, this HE is functionally unresponsive to RA. Here we show that WNTd mesoderm, before HE specification, is actually composed of two distinct KDR+ CD34neg populations. CXCR4negCYP26A1+ mesoderm gives rise to HOXA+ multilineage definitive HE in an RA-independent manner, whereas CXCR4+ ALDH1A2+ mesoderm gives rise to HOXA+ multilineage definitive HE in a stage-specific, RA-dependent manner. Furthermore, both RA-independent (RAi) and RA-dependent (RAd) HE harbour transcriptional similarity to distinct populations found in the early human embryo, including HSC-competent HE. This revised model of human haematopoietic development provides essential resolution to the regulation and origins of the multiple waves of haematopoiesis. These insights provide the basis for the generation of specific haematopoietic populations, including the de novo specification of HSCs.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
-
FC/FACS
-
Cell Biology
-
Stem Cells and Developmental Biology
In Development (Cambridge, England) on 15 April 2022 by Bredemeyer, A. L., Amrute, J. M., et al.
Tissue-resident macrophages are increasingly recognized as important determinants of organ homeostasis, tissue repair, remodeling and regeneration. Although the ontogeny and function of tissue-resident macrophages has been identified as distinct from postnatal hematopoiesis, the inability to specify, in vitro, similar populations that recapitulate these developmental waves has limited our ability to study their function and potential for regenerative applications. We took advantage of the concept that tissue-resident macrophages and monocyte-derived macrophages originate from distinct extra-embryonic and definitive hematopoietic lineages to devise a system to generate pure cultures of macrophages that resemble tissue-resident or monocyte-derived subsets. We demonstrate that human pluripotent stem cell-derived extra-embryonic-like and intra-embryonic-like hematopoietic progenitors differentiate into morphologically, transcriptionally and functionally distinct macrophage populations. Single-cell RNA sequencing of developing and mature cultures uncovered distinct developmental trajectories and gene expression programs of macrophages derived from extra-embryonic-like and intra-embryonic-like hematopoietic progenitors. These findings establish a resource for the generation of human tissue resident-like macrophages to study their specification and function under defined conditions and to explore their potential use in tissue engineering and regenerative medicine applications.
© 2022. Published by The Company of Biologists Ltd.
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
In Nat Cell Biol on 1 August 2023 by Ghersi, J. J., Baldissera, G., et al.
Fig.4.H

-
FC/FACS
-
Collected and cropped from Nat Cell Biol by CiteAb, provided under a CC-BY license
Image 1 of 1