Central nervous system (CNS) involvement and/or relapse remains one of the most important causes of morbidity/mortality in paediatric B-cell precursor acute lymphoblastic leukaemia (BCP-ALL) patients. To identify novel therapeutic targets and develop less aggressive therapies, a better understanding of the cellular and molecular microenvironment in leptomeningeal metastases is key. Here, we aimed to investigate the formation of metastatic leptomeningeal aggregates and their relevance to the expansion, survival and chemoresistance acquisition of leukaemia cells.
We used BCP-ALL xenograft mouse models, combined with immunohistofluorescence and flow cytometry, to study the development of CNS metastasis and the contribution of leptomeningeal cells to the organisation of leukaemic aggregates. To in vitro mimic the CNS metastasis, we established co-cultures of three-dimensional (3D) ALL cell spheroids and human leptomeningeal cells (hLMCs) and studied the effects on gene expression, proliferation, cytokine production, and chemoresistance.
In xenografted mice, ALL cells infiltrated the CNS at an early stage and, after crossing an ER-TR7+ fibroblast-like meningeal cell layer, they proliferated extensively and formed large vascularised leukaemic aggregates supported by a network of podoplanin+ leptomeningeal cells. In leukaemia spheroid-hLMC co-cultures, unlike conventional 2D co-cultures, meningeal cells strongly promoted the proliferation of leukaemic cells and generated a pro-inflammatory microenvironment. Furthermore, in 3D cell aggregates, leukaemic cells also developed chemoresistance, at least in part due to ABC transporter up-regulation.
Our results provide evidence for the formation of metastatic ALL-leptomeningeal cell aggregates, their pro-inflammatory profile and their contribution to leukaemic cell expansion, survival and chemoresistance in the CNS.
© 2025. The Author(s).
Product Citations: 279
In Cellular Oncology (Dordrecht) on 1 June 2025 by Ortiz-Sánchez, P., González-Soto, S., et al.
-
FC/FACS
-
Cancer Research
The tumor suppressor SALL2 opposes chemotherapeutic resistance in breast cancer.
In Molecular and Cellular Biochemistry on 1 May 2025 by Li, Q., Li, C., et al.
Chemotherapy continues to be the primary treatment for certain types of breast cancer. However, despite an initial positive response to chemotherapeutic agents, the development of resistance is inevitable. The exact molecular mechanisms underlying this phenomenon remain unclear. In this research, a significant downregulation of SALL2 expression was observed in chemo-resistant breast cancer, which was attributed to promoter methylation. Decreased SALL2 expression correlated significantly with poorer relapse-free survival in chemotherapy-treated patients with breast cancer. Functionally, SALL2 silencing induced a stem cell-like phenotype in breast cancer cells, fostering resistance to cisplatin both in vitro and in vivo. This resistance was mediated, at least in part, through the transcriptional regulation of BTG2, a negative regulator of stemness, achieved by direct binding to its promoter regions. These findings underscore the critical role of SALL2 in modulating cisplatin response and propose SALL2 as a potential prognostic biomarker for chemotherapy response in breast cancer.
© 2024. The Author(s).
-
Biochemistry and Molecular biology
-
Cancer Research
In Scientific Reports on 22 February 2025 by Lee, J., Min, H. K., et al.
This study aimed to investigate the therapeutic effect of human nasal turbinate-derived stem cells (hNTSCs) on mice with rheumatoid arthritis (RA) and identify hNTSC gene signatures with therapeutic effects on RA. hNTSCs were obtained from 20 healthy controls (HCs) who had undergone nasal turbinate surgery. Collagen-induced arthritis (CIA) mice were used to investigate the therapeutic effects of hNTSCs. The engraftment and migration abilities of hNTSCs were evaluated. CD4+CD25- T cells were co-cultured with hNTSCs, and effector T cell proliferation was evaluated by flow cytometry. Osteoclast differentiation was evaluated using mouse bone marrow monocytes which were cultured with M-CSF and RANKL, then TRAP staining was performed to measure effect of hNTSCs on osteoclastogenesis. Microarray assays were performed to identify gene expression differences between hNTSCs with CIA mice therapeutic or not and were validated by RT-qPCR. hNTSCs differentiated well into osteoblasts and adipocytes and expressed high levels of CXCL1 and osteoprotegerin. Single-cell RNA sequencing showed that hNTSCs clustered into 11 cell types, and cell surface markers were compatible with mesenchymal stem cells. hNTSC-treated CIA mice showed reductions in arthritis severity scores and incidence of arthritis. In engraft measurements, hNTSCs survived for 8 to 12 weeks in mice paws. Chemokine receptors expression increased in hNTSCs by IL-1β or TNF-α stimulation. CD4+CD25- T cell proliferation was reduced by hNTSCs and reversed by adding 1-MT (indoleamine 2,3-dioxygenase inhibitor), indicating that indoleamine 2,3-dioxygenase mediated T cell suppression. Osteoclastogenesis was suppressed by hNTSCs, and this was attenuated by anti-OPG Ab. hNTSCs therapeutic in CIA mice showed specific gene signatures with up-regulated genes (KRTAP1-5, HAS2, and CXCL1) and down-regulated genes (GSTT2B and C4B) compared to hNTSCs without CIA therapeutic effects. hNTSCs exhibited therapeutic potential in RA. Therapeutic effects were mediated by effector helper T cell suppression and the inhibition of osteoclastogenesis. In addition, hNTSCs with greater therapeutic effects on RA showed significant differences in their gene signatures.
© 2025. The Author(s).
-
ICC-IF
-
Homo sapiens (Human)
-
Stem Cells and Developmental Biology
The uptake of metallic nanoparticles in breast cancer cell lines is modulated by the HA-CD44 axis.
Preprint on BioRxiv : the Preprint Server for Biology on 17 February 2025 by Hullo, M., Mathé, C., et al.
Radio-enhancement is a promising anti-cancer approach based on a local radiation dose increase due to the presence of metallic nanoparticles (NPs) within cancer cells. Depending on their composition, size and cellular properties, NPs can follow multiple cellular pathways and entry routes. We observed that gold, platinum and TiO2 NPs are internalized at higher levels in mesenchymal cells compared to epithelial cells in breast cancer models. A global survey of gene expression between epithelial and mesenchymal cells exposed to 4 different NP types revealed an involvement of membrane structure, and further experiments confirmed that the hyaluronic acid (HA) and its receptor CD44 are mediators of metallic NP uptake into cells. We extended our results to a larger panel of breast cancer cell lines and again showed a preferential uptake of all NPs tested in mesenchymal cells and relying on the HA/CD44 axis. These data provide new considerations for the design of NP-based therapies targeting mesenchymal cancer cells, which are often resistant to treatment and correlate with poor prognosis and tumor recurrence.
-
Cancer Research
In Frontiers in Pharmacology on 29 October 2024 by Yu, L., Zang, C., et al.
Breast cancer continues to be a major health concern and is currently the most commonly diagnosed cancer worldwide. Relapse, metastasis, and therapy resistance are major clinical issues that doctors need to address. We believe BYL-719, which is PI3 kinase p110а inhibitor, could also inhibit the breast cancer stem cell phenotype and epithelial-to-mesenchymal transition (EMT). In addition to the PI3K/AKT signaling pathway, BYL-719 can also inhibit essential cancer-related signaling pathways, all of which would ultimately act on the microenvironment of cancer stem cells, which is quite complicated and regulates the characteristics of tumors. These include the stemness and resistance of malignant tumors, plasticity of cancer stem cells, and anti-apoptotic features.
A three-dimensional (3D) mammosphere culture method was used in vitro to culture and collect breast cancer stem cells (BCSCs). MTT, clonogenic, and cell apoptosis assays were used to detect cell viability, self-renewal, and differentiation abilities. A sphere formation assay under 3D conditions was used to detect the mammophore inhibition rate of BYL-719. The subpopulation of CD44+CD24- was detected using flow cytometry analysis while EMT biomarkers and essential signaling pathways were detected using western blotting. All the data were analyzed using GraphPad Prism 9 software.
BCSC-like cells were obtained by using the 3D cell culture method in vitro. We confirmed that BYL-719 could inhibit BCSC-like cell proliferation in 3D cultures and that the stemness characteristics of BCSC-like cells were inhibited. The PI3K/AKT/mTOR signaling pathway could be inhibited by BYL-719, and the Notch, JAK-STAT and MAPK/ERK signaling pathways which have crosstalk in the tumor microenvironment (TME) are also inhibited. By comparing eribulin-resistant breast cancer cell lines, we confirmed that BYL-719 could effectively overcome drug resistance.
The 3D cell culture is a novel and highly effective method for enriching BCSCs in vitro. Furthermore, the stemness and EMT of BCSCs were inhibited by BYL-719 by acting on various signaling pathways. Finally, we believe that drug resistance can be overcome by targeting the BCSCs. Conjugation of BYL-719 with other anti-neoplastic agents may be a promising treatment for this in clinic.
Copyright © 2024 Yu, Zang, Ye, Liu and Eucker.
-
FC/FACS
-
Homo sapiens (Human)
-
Cancer Research
-
Pharmacology
-
Stem Cells and Developmental Biology
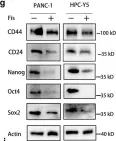
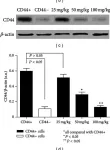
In Cell Biosci on 24 September 2023 by Xu, X., Ding, Y., et al.
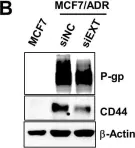
Fig.2.G

-
WB
-
Collected and cropped from Cell Biosci by CiteAb, provided under a CC-BY license
Image 1 of 14
In Sci Rep on 17 May 2019 by Hocevar, B. A.
Fig.3.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 14
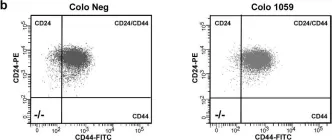
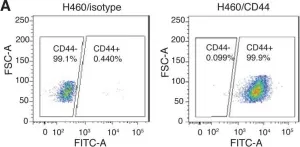
In Mol Oncol on 1 February 2019 by Parameswaran, S., Vizeacoumar, F. S., et al.
Fig.5.B

-
FC/FACS
-
Collected and cropped from Mol Oncol by CiteAb, provided under a CC-BY license
Image 1 of 14
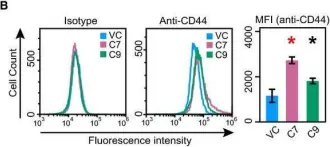
In Oncotarget on 19 September 2017 by Manandhar, S., Kim, C. G., et al.
Fig.1.G

-
WB
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 14
In Oncotarget on 19 September 2017 by Manandhar, S., Kim, C. G., et al.
Fig.3.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 14
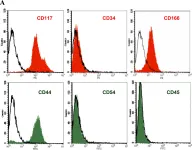
In J Transl Med on 27 January 2015 by Ghiabi, P., Jiang, J., et al.
Fig.1.C

-
IHC
-
Homo sapiens (Human)
Collected and cropped from J Transl Med by CiteAb, provided under a CC-BY license
Image 1 of 14
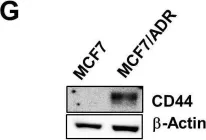
In Oncotarget on 30 October 2014 by Kumar, S., Kumar, D., et al.
Fig.7.A

-
ICC-IF
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 14
In Oncotarget on 30 October 2014 by Kumar, S., Kumar, D., et al.
Fig.8.B

-
ICC-IF
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 14
In PLoS One on 7 March 2014 by Shi, Y., Liu, C., et al.
Fig.4.A

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 14
In Evid Based Complement Alternat Med on 28 May 2013 by Yan, B., Zhou, Y., et al.
Fig.6.B

-
WB
-
Collected and cropped from Evid Based Complement Alternat Med by CiteAb, provided under a CC-BY license
Image 1 of 14
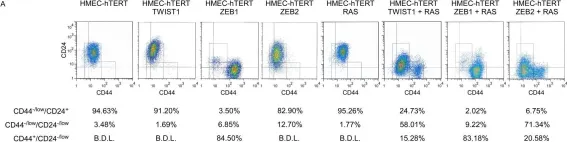
In PLoS Genet on 2 June 2012 by Morel, A. P., Hinkal, G. W., et al.
Fig.5.A

-
FC/FACS
-
Collected and cropped from PLoS Genet by CiteAb, provided under a CC-BY license
Image 1 of 14
In PLoS One on 1 June 2011 by Chen, J., Lu, Z., et al.
Fig.2.A

-
FC/FACS
-
Sus scrofa domesticus (Domestic pig)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 14
In BMC Cancer on 6 August 2010 by Bhat-Nakshatri, P., Appaiah, H., et al.
Fig.4.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from BMC Cancer by CiteAb, provided under a CC-BY license
Image 1 of 14
In BMC Cancer on 6 August 2010 by Bhat-Nakshatri, P., Appaiah, H., et al.
Fig.5.C

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from BMC Cancer by CiteAb, provided under a CC-BY license
Image 1 of 14