Osteoarthritis (OA) is a common degenerative disease caused by multiple pathological mechanisms wherein subchondral bone malfunction plays a substantial role. Recently, subchondral (SC) injection of orthobiologics has been attracting growing interest albeit the mainstream delivery method of mesenchymal stem cells (MSCs) is through intra-articular (IA). This study investigates the effect of SC injection of human umbilical cord mesenchymal stem cells (UCMSCs) on OA and its possible therapeutic mechanism compared to IA injection.
Male Sprague-Dawley rats with anterior cruciate ligament transection (ACLT) received saline or UCMSC injections via SC or IA. Consecutive injections once a week for three weeks and withdrawal for another four weeks, followed by Radiographical scanning, histopathological, immunohistochemical, and terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labelling (TUNEL) staining. Cell counting Kit-8 (CCK-8) assay, alkaline phosphatase (ALP), alizarin red staining (ARS), TUNEL, flow cytometry, quantitative real-time polymerase chain reaction (qRT-PCR) and Western blotting were employed in TNFα-induced MC3T3-E1 cells to illustrate the exact pathogenesis mechanism.
IA and SC UCMSC injections preserved cartilage, synovium, and subchondral bone parameters like trabecular bone volume fraction (BV/TV). SC injection uniquely improved Trabecular separation (Tb.Sp) and Trabecular number (Tb.N). SC and IA injections of UCMSCs demonstrated equivalent efficacy in promoting osteoblastic bone formation and attenuating aberrant angiogenesis of subchondral bone. In addition, we demonstrated that osteoblast apoptosis and Smad2-dependent TGF-beta (TGF-β) are crucial and interactive subchondral bone pathological features in OA. In vivo and vitro studies further revealed that UCMSCs inhibited excessive TGF-β/pSmad2 signaling to regulate aberrant vascularization, osteoblast apoptosis and differentiation imbalance, ultimately maintaining osteochondral homeostasis.
The efficacy of UCMSCs for treating OA rats via SC injection was equivalent to that of IA; and even superior to IA in terms of subchondral bone phenotype via regulating apoptosis and TGF-β/pSmad2 signaling in osteoblasts, suggesting SC injection of UCMSCs as a potential and promising cell therapy for OA treatment.
© 2025. The Author(s).
Product Citations: 330
In Stem Cell Research & Therapy on 9 May 2025 by Wu, C., Xu, H., et al.
-
Stem Cells and Developmental Biology
In Frontiers in Nutrition on 8 May 2025 by Lian, J., Men, Z., et al.
Folic acid has been associated with fetal development, especially in fetal immunity. Therefore, limited evidence regarding the effects of different folic acid supplementation of hepatitis B surface antigen (HBsAg) positive mothers in innate immunity in offspring. Herein, this study aimed to explore the association between folic acid supplementation and the innate immunity of neonates and the immunological efficacy of hepatitis B vaccine (HepB), which may provide insights that could inform pre-pregnancy health management in HBsAg-positive mothers.
It is an ambispective cohort study with 293 pairs of HBsAg-positive mothers-offspring in Taiyuan, Shanxi Province, China. Mothers were classified into three groups according to the time of starting folic acid supplementation, non-supplementation group, pre-pregnancy group and post-pregnancy supplementation group. Immunological indexes such as immune cells proportion and innate immune mediators in cord blood and anti-HBs in infants were measured. Differences in immunological indexes were analyzed by One-Way ANOVA test. Univariate and multivariate analyses were performed for factors associated with abnormal immunological indexes and potential confounders were adjusted.
The preconception folic acid group showed a significantly higher expression levels of STING (P = 0.005) and pNF-κB (P = 0.010) in cord blood along with higher anti-HBs titres (P = 0.006), when compared to both non-supplementation group and post-pregnancy supplementation group. Higher anti-HBs levels indicate a stronger immune response to HepB and may enhance protection against HBV infection during early life. Infants in the high pNF-κB expression group exhibited a significantly elevated seropositive rate of HepB compared to those in the low pNF-κB expression group (P = 0.037). There were no mediation effects and no moderation effects in this study, potentially due to the direct influence of folic acid supplementation on immune responses or the limited sample size.
In conclusion, our findings demonstrate that preconception folic acid supplementation may enhance HepB vaccine responsiveness in infants of HBsAg-positive mothers. Meanwhile, high pNF-κB expression in cord blood can increase seropositive rates in infants. This discovery has significant public health implications, as it may provide a simple and accessible intervention to improve vaccination outcomes and reduce HBV transmission in endemic regions.
Copyright © 2025 Lian, Men, Xu, Li, Li, Wang, Yao, Li, Qu, Feng and Wang.
-
Immunology and Microbiology
Perturbing LSD1 and WNT rewires transcription to synergistically induce AML differentiation.
In Nature on 16 April 2025 by Hosseini, A., Dhall, A., et al.
Impaired differentiation is a hallmark of myeloid malignancies1,2. Therapies that enable cells to circumvent the differentiation block, such as all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), are by and large curative in acute promyelocytic leukaemia3, but whether 'differentiation therapy' is a generalizable therapeutic approach for acute myeloid leukaemia (AML) and beyond remains incompletely understood. Here we demonstrate that simultaneous inhibition of the histone demethylase LSD1 (LSD1i) and the WNT pathway antagonist GSK3 kinase4 (GSK3i) robustly promotes therapeutic differentiation of established AML cell lines and primary human AML cells, as well as reducing tumour burden and significantly extending survival in a patient-derived xenograft mouse model. Mechanistically, this combination promotes differentiation by activating genes in the type I interferon pathway via inducing expression of transcription factors such as IRF7 (LSD1i) and the co-activator β-catenin (GSK3i), and their selective co-occupancy at targets such as STAT1, which is necessary for combination-induced differentiation. Combination treatment also suppresses the canonical, pro-oncogenic WNT pathway and cell cycle genes. Analysis of datasets from patients with AML suggests a correlation between the combination-induced transcription signature and better prognosis, highlighting clinical potential of this strategy. Collectively, this combination strategy rewires transcriptional programs to suppress stemness and to promote differentiation, which may have important therapeutic implications for AML and WNT-driven cancers beyond AML.
© 2025. The Author(s).
-
Biochemistry and Molecular biology
In Life (Basel, Switzerland) on 28 January 2025 by Saino, O., Ogawa, Y., et al.
The efficacy of hematopoietic stem cell (HSC) therapy for cerebral infarction has been previously demonstrated. However, the lack of response in some patients has hindered its widespread use. To establish HSC therapy as a standard treatment, it is important to examine the causes of non-responsiveness. In this study, we aimed to identify the specifications of transplanted cells based on their therapeutic mechanisms to predict treatment success. We found that HSC therapy activates injured cerebral endothelial cells via gap junctions because cell adhesion between HSCs and the endothelium plays an essential role in cellular communication via gap junctions. The expression of the adhesion molecule integrin β2 (CD18) in CD34-positive (CD34+) cells was identified as critical for the therapeutic effect on cerebral infarction in a murine model. Cells with low CD18 expression exhibited a weaker therapeutic effect than cells with high CD18 expression, even when the same number of HSCs was administered. The expression of CD18 in CD34+ cells can be used as a specification marker for transplanted HSCs and is useful for identifying non-responders. Furthermore, quantification of CD18 expression is crucial for evaluating the cellular potential of cell-based therapies for diseases where therapeutic effects are mediated through cell adhesion.
-
Stem Cells and Developmental Biology
Identification of human cranio-maxillofacial skeletal stem cells for mandibular development.
In Science Advances on 3 January 2025 by Wang, Z., Wang, K., et al.
Compared with long bone that arises from the mesoderm, the major portion of the maxillofacial bones and the front bone of the skull are derived from cranial neural crest cells and undergo intramembranous ossification. Human skeletal stem cells have been identified in embryonic and fetal long bones. Here, we describe a single-cell atlas of the human embryonic mandible and identify a population of cranio-maxillofacial skeletal stem cells (CMSSCs). These CMSSCs are marked by interferon-induced transmembrane protein 5 (IFITM5) and are specifically located around the periosteum of the jawbone and frontal bone. Additionally, these CMSSCs exhibit strong self-renewal and osteogenic differentiation capacities but lower chondrogenic differentiation potency, mediating intramembranous bone formation without cartilage formation. IFITM5+ cells are also observed in the adult jawbone and exhibit functions similar to those of embryonic CMSSCs. Thus, this study identifies CMSSCs that orchestrate the intramembranous ossification of cranio-maxillofacial bones, providing a deeper understanding of cranio-maxillofacial skeletal development and promising seed cells for bone repair.
-
Stem Cells and Developmental Biology
In PLoS One on 1 June 2011 by Chen, J., Lu, Z., et al.
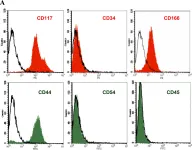
Fig.2.A

-
FC/FACS
-
Sus scrofa domesticus (Domestic pig)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 1