Adipose-derived stromal/stem cells (ASCs) are promising for the treatment of many diseases, including tissue injury or degeneration associated with serious disease and high morbidity. The extent of cell therapy effectiveness, however, may be limited by the lower survival of implanted cells in environments of tissue damage. Therefore, strategies to improve cell survival are important, such as by pretreating/preconditioning cells with a beneficial agent. We investigated the pretreatment of human ASCs (hASCs) with StemRegenin 1 (SR1), a purine derivative used in clinical protocols for in vitro hematopoietic stem/progenitor cell expansion. We pretreated hASCs with SR1 and analyzed the resulting cells (SR1-hASCs) as compared to non-treated cells (NT-hASCs). We noted that treatment with SR1 significantly increased the proliferation and migration of hASCs, as well as their secretion of paracrine factors of interest, and did not affect their cell differentiation capacity. Furthermore, when these SR1-hASCs were subsequently exposed to antimycin A, a mitochondrial respiratory chain inhibitor, they showed significantly higher antioxidative, anti-apoptotic, and pro-survival abilities as compared to NT-hASCs. Since oxidative stress and other harsh environments result from tissue damage, our results support that the preconditioning of hASCs with SR1 may enhance their protective, reparative, and regenerative, and thus therapeutic, efficacy.
© 2025 The Authors.
Product Citations: 217
In Molecular Therapy. Methods Clinical Development on 11 December 2025 by Zhao, J., Yu, B., et al.
-
Stem Cells and Developmental Biology
Expression of intron-containing HIV-1 RNA induces NLRP1 inflammasome activation in myeloid cells.
In PLoS Biology on 1 September 2025 by Jalloh, S., Hughes, I. K., et al.
Despite the success of antiretroviral therapy in suppressing plasma viremia in people living with human immunodeficiency virus type-1 (HIV-1), persistent viral RNA expression in tissue reservoirs is observed and can contribute to HIV-1-induced immunopathology and comorbidities. Infection of long-lived innate immune cells, such as tissue-resident macrophages and microglia may contribute to persistent viral RNA production and chronic inflammation. We recently reported that de novo cytoplasmic expression of HIV-1 intron-containing RNA (icRNA) in macrophages and microglia leads to MDA5 and MAVS-dependent innate immune sensing and induction of type I IFN responses, demonstrating that HIV icRNA is a pathogen-associated molecular pattern (PAMP). In this report, we show that cytoplasmic expression of HIV-1 icRNA also induces NLRP1 inflammasome activation and IL-1β secretion in macrophages and microglia in an RLR- and endosomal TLR-independent manner. Infection of both macrophages and microglia with either replication-competent or single-cycle HIV-1 induced IL-1β secretion, which was attenuated when cytoplasmic expression of viral icRNA was prevented. While IL-1β secretion was blocked by treatment with caspase-1 inhibitors or knockdown of NLRP1 or caspase-1 expression in HIV-infected macrophages, overexpression of NLRP1 significantly enhanced IL-1β secretion in an HIV-icRNA-dependent manner. Immunoprecipitation analysis revealed interaction of HIV-1 icRNA, but not multiply-spliced HIV-1 RNA, with NLRP1, suggesting that HIV-1 icRNA sensing by NLRP1 is sufficient to trigger inflammasome activation. Together, these findings reveal a pathway of NLRP1 inflammasome activation induced by de novo expressed HIV icRNA in HIV-infected myeloid cells.
Copyright: © 2025 Jalloh et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
-
Genetics
In BMC Cancer on 1 July 2025 by Xu, H., Cao, N., et al.
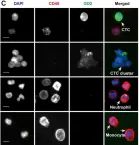
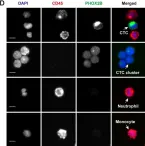
The clinical significance of circulating tumor cell (CTC) clusters in highly metastatic tumors hasn't yet been revealed. Here, we demonstrated the diagnostic and prognostic value of CTC clusters in neuroblastoma (NB) which is the most prevalent childhood extracranial solid tumor.
We employed cascaded filter deterministic lateral displacement microfluidic chips to enrich CTCs and CTC clusters in 64 newly diagnosed NB patients. CTCs and CTC clusters were identified by CD45-, GD2+/PHOX2B+, DAPI + immunofluorescence staining, with cells displaying characteristic neoplastic morphology.
Among NB patients, 85.94% and 50.00% were positive for CTCs and CTC clusters, respectively; no CTCs or CTC clusters were detected in healthy children. Moreover, CTC and CTC cluster numbers differed significantly across different primary sites, clinical and pathologic features, and risk stratifications, while no significant differences in CTC and CTC cluster counts were observed in relation to sex, age, and MYCN gene amplification. CTCs and CTC clusters indicated metastasis and strongly correlated with minimal residual disease. Of note, CTC clusters ≥ 2.5/2 mL were closely associated with bone marrow metastasis and demonstrated significant differences in the hazard ratio of overall survival.
CTCs and CTC clusters are sensitive non-invasive biomarkers for NB diagnosis and prognosis, especially the prominent role in tumor emergencies. CTC clusters closely correlate with bone marrow metastasis and represent promising indicator for the monitoring of metastasis in NB emergencies. The mechanisms of CTC cluster formation and their specific role in the metastasis cascade deserve further elucidation which may serve as targets to inhibit NB bone marrow metastasis.
© 2025. The Author(s).
-
ICC-IF
-
Cancer Research
Efficacy of NAMPT inhibition in T-cell acute lymphoblastic leukemia.
In PLoS ONE on 17 June 2025 by Vrana, C., Zhang, M., et al.
Novel agents targeting upregulated signaling pathways are needed to improve outcomes in T-cell acute lymphoblastic leukemia (T-ALL), since conventional cytotoxic chemotherapy regimens have reached the limits of tolerability. We identified upregulated, targetable signaling pathways common to both human T-ALL samples and a KrasLSL-G12D/+.Mb1Cre/+ murine model of T-ALL. We found the NAMPT inhibitor FK866 had the greatest cytotoxicity of a panel of small molecule inhibitors tested in human and mouse T-ALL cell lines, and in patient derived xenograft (PDX)-expanded T-ALL patient samples. We subsequently tested FK866 in vivo in PDX mouse models of T-ALL, and found that it significantly reduced the peripheral blood disease burden and prolonged the survival of leukemic mice (median survival of 60.5 vs 21 days, p = 0.0007). This screen for targetable pathways in T-ALL generated in vitro and in vivo preclinical data supporting NAMPT inhibition as a promising strategy for the treatment of T-ALL.
Copyright: © 2025 Vrana et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
Novel PAP-targeted CAR-T therapy enhances antitumor efficacy through CoupledCAR approach.
In Journal for Immunotherapy of Cancer on 31 May 2025 by Cao, Z., Pu, C., et al.
The challenges that remain in the treatment of solid tumors with chimeric antigen receptor (CAR)-T cells include limited solid tumor-specific targets and poor CAR-T cell expansion and function due to limited availability of solid tumor antigens outside the tumor microenvironment. Prostate cancer is the second most common cancer among men worldwide. Current CAR-T therapies for prostate cancer lack specific targets, posing safety risks. To overcome these problems, we identified prostatic acid phosphatase (PAP, also known as ACPP or ACP3) as a feasible CAR-T target for prostate cancer and developed CoupledCAR, a novel approach for expanding tumor-targeting CAR-T cells without tumor antigens.
We analyzed the expression of PAP from The Cancer Genome Atlas database and validated its expression in normal and cancer tissues through immunohistochemistry staining. To generate anti-PAP specific antibodies, we screened the human single-chain antibody library using transmembrane PAP-His antigen and selected antibodies based on their binding ability and specificity. We constructed PAP-targeted CAR and evaluated their antitumor efficacy both in vitro and in vivo. We validated the function of PAP CoupledCAR in both in vitro and in vivo experiments, and further analyzed its mechanism using single-cell RNA sequencing (scRNA-Seq).
PAP was specifically expressed in prostate epithelial and prostate cancer cells, with no expression in other tissues. Seven single-chain variable fragments were screened from the human single-chain antibody library, with S5D1 showing the highest binding ability for the PAP. PAP CAR-T cells demonstrated strong antitumor efficacy both in vitro and in vivo. Furthermore, the CoupledCAR system significantly expanded PAP CAR-T cells, promoting memory-like status, reducing exhaustion, and enhancing their antitumor efficacy. The scRNA-Seq demonstrated that the expansion of PAP CAR-T cells in the CoupledCAR system is mediated by costimulatory signals and cytokine signals, rather than T-cell receptor signals.
Our study is the first to demonstrate that PAP is a specific target for CAR-T therapy in prostate cancer, both in vitro and in vivo. We developed the CoupledCAR platform technology for solid tumor CAR-T cell therapy, enabling the expansion of tumor-targeting CAR-T cells without requiring tumor antigens and thereby enhancing their functionality against solid tumors.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
In BMC Cancer on 1 July 2025 by Xu, H., Cao, N., et al.
Fig.1.C
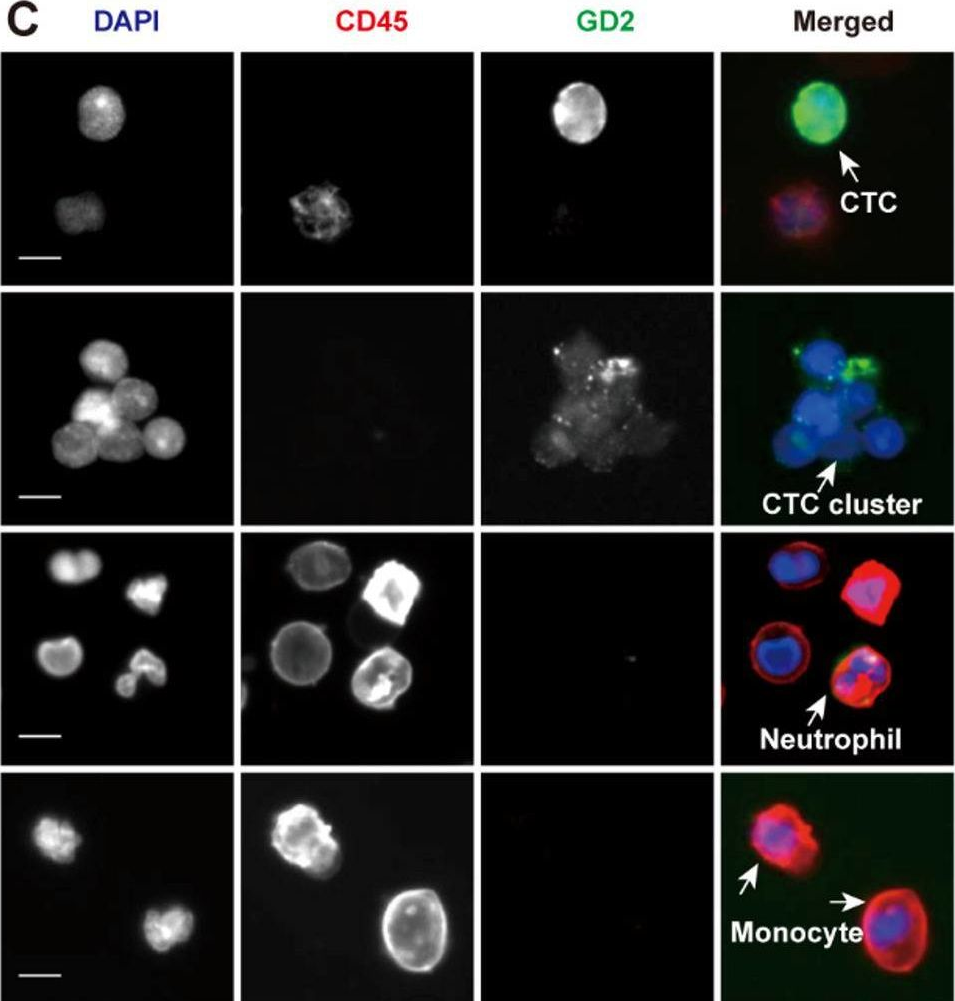
-
ICC-IF
-
Collected and cropped from BMC Cancer by CiteAb, provided under a CC-BY license
Image 1 of 2
In BMC Cancer on 1 July 2025 by Xu, H., Cao, N., et al.
Fig.1.D

-
ICC-IF
-
Collected and cropped from BMC Cancer by CiteAb, provided under a CC-BY license
Image 1 of 2