Background Human muscle reserve cells (MuRC) represent a quiescent MuSC population generated in vitro that exhibit heterogeneous Pax7 expression, with a Pax7 High subset in a deeper quiescent state. However, conventional identification of Pax7 High cells requires intracellular staining, limiting their viability for functional studies. This study investigates autofluorescence (AF) as a potential biomarker to identify functionally distinct human MuRC subpopulations. Methods Human myoblasts (MB) and MuRC were analysed for AF by fluorescence microscopy and flow cytometry. Cellular metabolic composition was assessed by NADH/NADPH quantification and lipid staining. Human MuRC subpopulations were sorted by AF intensity and analysed for Pax7 expression, cell cycle re-entry, proliferation, clonal expansion, and myogenic differentiation. In vivo transplantation of MuRC-AF High and MuRC-AF Low populations into immunodeficient mice assessed survival and regenerative potential using bioluminescence imaging and immunohistochemistry. Results Human MuRC showed a 3-fold increase in mean fluorescence intensity compared to MB, with AF peak at 405 nm excitation. Lipid staining revealed a 1.6-fold increase in lipid content in MuRC, while NADH/NADPH levels were similar between MB and MuRC. Flow cytometry identified MuRC-AF High as a Pax7 High -enriched subpopulation. Functionally, MuRC-AF High cells exhibited delayed cell cycle re-entry and slower proliferation but retained differentiation potential. In vivo , both MuRC-AF High and MuRC-AF Low survived transplantation with no significant differences in engraftment efficiency. They contributed to the generation of human Pax7 positives MuSC and both subpopulations retain their regenerative capacity upon re-injury. Conclusion AF allows the identification of human MuRC subsets, with the AF High subpopulation associated with increased lipid content. MuRC-AF High cells are enriched in Pax7 High cells and show delayed activation, slower proliferation and comparable engraftment efficiency to the AF Low subpopulation. These findings provide a novel perspective on AF as a potential biomarker to identify functionally distinct muscle progenitor subsets and highlight its relevance in muscle regeneration research.
Product Citations: 137
Preprint on BioRxiv : the Preprint Server for Biology on 28 March 2025 by Bouche, A., Michel, D., et al.
-
Stem Cells and Developmental Biology
Non-muscle myosin II inhibition at the site of axon injury increases axon regeneration.
In Nature Communications on 26 March 2025 by Heo, K., Ho, T. S., et al.
Motor axon regeneration following peripheral nerve injury is critical for motor recovery but therapeutic interventions enhancing this are not available. We conduct a phenotypic screen on human motor neurons and identified blebbistatin, a non-muscle myosin II inhibitor, as the most effective neurite outgrowth promotor. Despite its efficacy in vitro, its poor bioavailability limits in vivo application. We, therefore, utilize a blebbistatin analog, NMIIi2, to explore its therapeutic potential for promoting axon regeneration. Local NMIIi2 application directly to injured axons enhances regeneration in human motor neurons. Furthermore, following a sciatic nerve crush injury in male mice, local NMIIi2 administration to the axonal injury site facilitates motor neuron regeneration, muscle reinnervation, and functional recovery. NMIIi2 also promotes axon regeneration in sensory, cortical, and retinal ganglion neurons. These findings highlight the therapeutic potential of topical NMII inhibition for treating axon damage.
© 2025. The Author(s).
-
MACS
-
Homo sapiens (Human)
-
Neuroscience
In International Journal of Molecular Sciences on 6 November 2024 by Andrejkovits, Á. V., Hutanu, A., et al.
Studies suggest that the dynamic changes in cellular response might correlate with disease severity and outcomes in SARS-CoV-2 patients. The study aimed to investigate the dynamic changes of lymphocyte subsets in patients with COVID-19. In this regard, 53 patients with COVID-19 were prospectively included, classified as mild, moderate, and severe. The peripheral lymphocyte profiles (LyT, LyB, and NK cells), as well as CD4+/CD8+, CD3+/CD19+, CD3+/NK and CD19+/NK ratios, and their dynamic changes during hospitalization and correlation with disease severity and outcome were assessed. We found significant differences in CD3+ lymphocytes between severity groups (p < 0.0001), with significantly decreased CD3+CD4+ and CD3+CD8+ in patients with severe disease (p < 0.0001 and p = 0.048, respectively). Lower CD3+/CD19+ and CD3+/NK ratios among patients with severe disease (p = 0.019 and p = 0.010, respectively) were found. The dynamic changes of lymphocyte subsets showed a significant reduction in NK cells (%) and a significant increase in CD3+CD4+ and CD3+CD8+ cells in patients with moderate and severe disease. The ROC analysis on the relationship between CD3+ cells and fatal outcome yielded an AUC of 0.723 (95% CI 0.583-0.837; p = 0.007), while after addition of age and SpO2, ferritin and NLR, the AUC significantly improved to 0.927 (95%CI 0.811-0.983), p < 0.001 with a sensitivity of 90.9% (95% CI 58.7-99.8%) and specificity of 85.7% (95% CI 69.7-95.2%). The absolute number of CD3+ lymphocytes might independently predict fatal outcomes in COVID-19 patients and T-lymphocyte subset evaluation in high-risk patients might be useful in estimating disease progression.
-
COVID-19
In Frontiers in Oncology on 4 November 2024 by Guimarães, S. J. A., Vale, A. A. M., et al.
Penile squamous cell carcinoma (PSCC) is a largely neglected condition, predominantly affecting underdeveloped regions, and is associated with risk factors such as low socioeconomic status, phimosis, and human papillomavirus (HPV) infection. Unlike other urogenital cancers, its pathophysiology and therapeutic targets remain poorly understood, particularly regarding the immune response to the tumor microenvironment. This study aims to investigate immune cell infiltration profiles, dendritic cell maturation, and lymphocyte apoptosis in both HPV-positive and HPV-negative PSCC. Clinical and histopathological data, along with peripheral blood and tumor tissue samples, were collected from 30 patients (66.6% were HPV-positive and 33.3% HPV-negative), with an additional 19 healthy donors serving as controls. Tumor-infiltrating immune cells were analyzed following enzymatic digestion of tumor tissue, enabling detailed phenotypic characterization. A simulated tumor microenvironment was created using supernatants derived from primary cultures of HPV-positive PSCC tumors. Peripheral blood mononuclear cells were isolated and differentiated into dendritic cells (Mo-DCs) for further phenotyping and lymphoproliferation assays. Lymphocytes from healthy donors and patients were exposed to tumor culture supernatants to evaluate apoptosis induced by the tumor microenvironment. Results showed that HPV-positive tumors exhibited lower T lymphocyte frequencies compared to HPV-negative tumors. Additionally, patients infected with high-risk HPV demonstrated reduced maturation rates of Mo-DCs and decreased expression of co-stimulatory molecules on these cells compared to healthy donors. Furthermore, Mo-DCs from hrHPV-positive patients showed impaired lymphoproliferation capacity relative to controls, while HPV-negative patients exhibited a trend towards reduced lymphoproliferative ability. Regarding the simulated tumor microenvironment, lymphocytes from healthy donors underwent apoptosis, contrasting with patients' lymphocytes, which showed increased viability when cultured with tumor supernatants. These results underscore the impact of HPV infection on T lymphocyte infiltration, Mo-DC maturation, and lymphocyte survival in PSCC, offering critical insights for advancing our understanding of the tumor microenvironment and guiding the development of immunotherapy strategies.
Copyright © 2024 Guimarães, Vale, Rocha, Butarelli, da Silva, Deus, Nogueira, Coelho, Pereira and Azevedo-Santos.
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
Cranioencephalic functional lymphoid units in glioblastoma.
In Nature Medicine on 1 October 2024 by Dobersalske, C., Rauschenbach, L., et al.
The ecosystem of brain tumors is considered immunosuppressed, but our current knowledge may be incomplete. Here we analyzed clinical cell and tissue specimens derived from patients presenting with glioblastoma or nonmalignant intracranial disease to report that the cranial bone (CB) marrow, in juxtaposition to treatment-naive glioblastoma tumors, harbors active lymphoid populations at the time of initial diagnosis. Clinical and anatomical imaging, single-cell molecular and immune cell profiling and quantification of tumor reactivity identified CD8+ T cell clonotypes in the CB that were also found in the tumor. These were characterized by acute and durable antitumor response rooted in the entire T cell developmental spectrum. In contrast to distal bone marrow, the CB niche proximal to the tumor showed increased frequencies of tumor-reactive CD8+ effector types expressing the lymphoid egress marker S1PR1. In line with this, cranial enhancement of CXCR4 radiolabel may serve as a surrogate marker indicating focal association with improved progression-free survival. The data of this study advocate preservation and further exploitation of these cranioencephalic units for the clinical care of glioblastoma.
© 2024. The Author(s).
In Oncotarget on 24 July 2018 by Onozawa, E., Shibayama, H., et al.
Fig.2.E

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 3
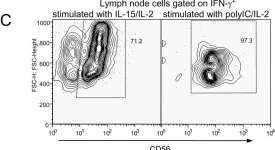
In PLoS One on 23 December 2009 by Kwant-Mitchell, A., Pek, E. A., et al.
Fig.5.C

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3
In PLoS One on 23 December 2009 by Kwant-Mitchell, A., Pek, E. A., et al.
Fig.9.A

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3