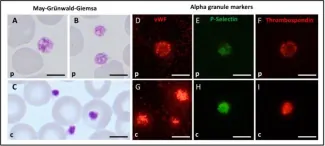
The GATA1 transcription factor is essential for normal erythropoiesis and megakaryocytic differentiation. Germline GATA1 pathogenic variants in the N-terminal zinc finger (N-ZF) are typically associated with X-linked thrombocytopenia, platelet dysfunction, and dyserythropoietic anemia. A few variants in the C-terminal ZF (C-ZF) domain are described with normal platelet count but altered platelet function as the main characteristic. Independently performed molecular genetic analysis identified a novel hemizygous variant (c.865C>T, p.H289Y) in the C-ZF region of GATA1 in a German patient and in a Spanish patient. We characterized the bleeding and platelet phenotype of these patients and compared these findings with the parameters of two German siblings carrying the likely pathogenic variant p.D218N in the GATA1 N-ZF domain. The main difference was profound thrombocytopenia in the brothers carrying the p.D218N variant compared to a normal platelet count in patients carrying the p.H289Y variant; only the Spanish patient occasionally developed mild thrombocytopenia. A functional platelet defect affecting αIIbβ3 integrin activation and α-granule secretion was present in all patients. Additionally, mild anemia, anisocytosis, and poikilocytosis were observed in the patients with the C-ZF variant. Our data support the concept that GATA1 variants located in the different ZF regions can lead to clinically diverse manifestations.
Product Citations: 16
In Cells on 14 October 2022 by Bastida, J. M., Malvestiti, S., et al.
-
ICC-IF
-
Homo sapiens (Human)
-
Cell Biology
In Blood Advances on 13 September 2022 by Palma-Barqueros, V., Revilla, N., et al.
Src-related thrombocytopenia (SRC-RT) is a rare autosomal dominant, inherited platelet disorder resulting from the p.E527K heterozygous germline gain-of-function variant of Src. To date, genetic diagnosis of the disease has only been reported in 7 patients from 3 unrelated families. The clinical features ranged from isolated thrombocytopenia to complex syndromic manifestations characterized by thrombocytopenia, bleeding, myelofibrosis, splenomegaly, and bone disease. We report a new 3-generation kindred with the Src p.E527K variant. Patients presented with rather variable platelet counts (38-139 × 109/L), mildly impaired platelet function, >15% immature platelet fraction, and with a significant proportion of large-giant platelets. Four adults from the family were diagnosed with immune thrombocytopenia (ITP) and underwent splenectomy, achieving sustained platelet counts >75 × 109/L for several years; increases in platelet counts were also observed after corticosteroid therapy. Four of 7 Src p.E527K variant carriers showed immune defects and recurrent infections. In addition, a range of neurological symptoms, from specific language impairment to epilepsy, was seen in some family members. Patient platelets exhibited constitutive Src, Bruton tyrosine kinase, and phospholipase Cγ2 activation, and after stimulating CD19 cells by crosslinking surface immunoglobulin M, phosphorylated extracellular signal-regulated kinase (ERK) was significantly increased in B cells from individuals carrying the Src p.E527K substitution. In summary, in addition to causing impaired platelet production, SRC-RT may associate immune dysregulation and increased platelet consumption. In families in whom several members are responsive to ITP-directed therapies, an underlying Src p.E527K variant should be excluded.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Biomedicines on 13 March 2021 by Runser, A., Schaning, C., et al.
Heparin-induced thrombocytopenia (HIT) is a thrombocytopenia caused by heparin and mediated by an atypical immune mechanism leading to a paradoxical high thrombotic risk, associated with severe morbidity or death. The diagnosis of HIT combines a clinical scoring of pretest probability and laboratory testing. First-line routine tests are antigen binding assays detecting specific antibodies. The most sensitive of these tests have a high HIT-negative predictive value enabling HIT diagnosis to be ruled out when negative. However, HIT-positive predictive value is low, and a functional assay evaluating the pathogenicity of the antibodies should be performed to exclude false-positive results. In contrast to screening assays, functional assays are highly specific but technically challenging, and are thus performed in referral laboratories, where platelet activation is detected using radioactive serotonin (serotonin release assay, SRA) or visually (heparin-induced platelet activation, HIPA). Flow cytometry is a possible alternative. It is, however, currently not widely used, mostly because of the lack of standardization of the published assays. This article describes and discusses the standardization of a HIT flow cytometry assay (HIT-FCA) method, which subsequently led to the development and commercialization of a CE-marked assay (HIT Confirm®, Emosis, France) as a suitable rapid HIT functional test.
Platelets expressing IgG receptor FcγRIIA/CD32A determine the severity of experimental anaphylaxis.
In Science Immunology on 13 April 2018 by Beutier, H., Hechler, B., et al.
Platelets are key regulators of vascular integrity; however, their role in anaphylaxis, a life-threatening systemic allergic reaction characterized by the loss of vascular integrity and vascular leakage, remains unknown. Anaphylaxis is a consequence of inappropriate cellular responses triggered by antibodies to generally harmless antigens, resulting in a massive mediator release and rapidly occurring organ dysfunction. Human platelets express receptors for immunoglobulin G (IgG) antibodies and can release potent mediators, yet their contribution to anaphylaxis has not been previously addressed in mouse models, probably because mice do not express IgG receptors on platelets. We investigated the contribution of platelets to IgG-dependent anaphylaxis in human IgG receptor-expressing mouse models and a cohort of patients suffering from drug-induced anaphylaxis. Platelet counts dropped immediately and markedly upon anaphylaxis induction only when they expressed the human IgG receptor FcγRIIA/CD32A. Platelet depletion attenuated anaphylaxis, whereas thrombocythemia substantially worsened its severity. FcγRIIA-expressing platelets were directly activated by IgG immune complexes in vivo and were sufficient to restore susceptibility to anaphylaxis in resistant mice. Serotonin released by activated platelets contributed to anaphylaxis severity. Data from a cohort of patients suffering from drug-induced anaphylaxis indicated that platelet activation was associated with anaphylaxis severity and was accompanied by a reduction in circulating platelet numbers. Our findings identify platelets as critical players in IgG-dependent anaphylaxis and provide a rationale for the design of platelet-targeting strategies to attenuate the severity of anaphylactic reactions.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
In Scientific Reports on 10 July 2017 by Ma, R., Xie, R., et al.
The mechanisms that eliminate activated platelets in inflammation-induced disseminated intravascular coagulation (DIC) in micro-capillary circulation are poorly understood. This study explored an alternate pathway for platelet disposal mediated by endothelial cells (ECs) through phosphatidylserine (PS) and examined the effect of platelet clearance on procoagulant activity (PCA) in sepsis. Platelets in septic patients demonstrated increased levels of surface activation markers and apoptotic vesicle formation, and also formed aggregates with leukocytes. Activated platelets adhered were and ultimately digested by ECs in vivo and in vitro. Blocking PS on platelets or αvβ3 integrin on ECs attenuated platelet clearance resulting in increased platelet count in a mouse model of sepsis. Furthermore, platelet removal by ECs resulted in a corresponding decrease in platelet-leukocyte complex formation and markedly reduced generation of factor Xa and thrombin on platelets. Pretreatment with lactadherin significantly increased phagocytosis of platelets by approximately 2-fold, diminished PCA by 70%, prolonged coagulation time, and attenuated fibrin formation by 50%. Our results suggest that PS-mediated clearance of activated platelets by the endothelium results in an anti-inflammatory, anticoagulant, and antithrombotic effect that contribute to maintaining platelet homeostasis during acute inflammation. These results suggest a new therapeutic target for impeding the development of DIC.
In Cells on 14 October 2022 by Bastida, J. M., Malvestiti, S., et al.
Fig.4.A

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 1