Lung cancer is the leading cause of cancer mortality among people with HIV (PWH), with increased incidence and poor outcomes. This study explored whether the tumor microenvironment (TME) of HIV-associated non-small cell lung cancer (NSCLC) limits tumor-specific immune responses. With a matched cohort of NSCLC from PWH and people without HIV (PWOH), we used imaging mass cytometry, linear mixed effects model and AI-based pageRank mathematical algorithm based on spectral graph theory to demonstrate that HIV-associated tumors demonstrate differential distribution of tumor infiltrating CD8+ and CD4+ T cells, enriched for the expression of PD-1 and Lag-3, as well as activation and proliferation markers. We also demonstrate higher expression of immunoregulatory molecules (PD-L1, PD-L2, B7-H3, B7-H4, IDO1 and VISTA), among tumor-associated macrophages. Discrimination of cells between tumors from PWH versus PWOH was confirmed by spectral graph theory with 84.6% accuracy. Furthermore, we noted differences in spatial orientation of immune cells within the TME of PWH compared to PWOH. Additionally, cells from PWH, compared to PWOH, exhibited decreased tumor killing when exposed to HLA-matched NSCLC cell lines. In conclusion, our study demonstrates that the HIV-associated tumor microenvironment sustains a unique immune landscape, with evidence of immune cells with enhanced immunoregulatory phenotypes and impaired anti-tumor responses, with implications for response to immune checkpoint blocker therapies.
Product Citations: 66
In The Journal of Clinical Investigation on 3 June 2025 by Desai, S. S., Salahuddin, S., et al.
-
Cancer Research
-
Immunology and Microbiology
Autoregulated splicing of TRA2β programs T cell fate in response to antigen-receptor stimulation.
In Science on 13 September 2024 by Karginov, T. A., Menoret, A., et al.
T cell receptor (TCR) sensitivity to peptide-major histocompatibility complex (MHC) dictates T cell fate. Canonical models of TCR sensitivity cannot be fully explained by transcriptional regulation. In this work, we identify a posttranscriptional regulatory mechanism of TCR sensitivity that guides alternative splicing of TCR signaling transcripts through an evolutionarily ultraconserved poison exon (PE) in the RNA-binding protein (RBP) TRA2β in mouse and human. TRA2β-PE splicing, seen during cancer and infection, was required for TCR-induced effector T cell expansion and function. Tra2β-PE skipping enhanced T cell response to antigen by increasing TCR sensitivity. As antigen levels decreased, Tra2β-PE reinclusion allowed T cell survival. Finally, we found that TRA2β-PE was first included in the genome of jawed vertebrates that were capable of TCR gene rearrangements. We propose that TRA2β-PE splicing acts as a gatekeeper of TCR sensitivity to shape T cell fate.
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
Enhanced and sustained T cell activation in response to fluid shear stress.
In IScience on 21 June 2024 by Sarna, N. S., Desai, S. H., et al.
The efficacy of T cell therapies in treating solid tumors is limited by poor in vivo persistence, proliferation, and cytotoxicity, which can be attributed to limited and variable ex vivo activation. Herein, we present a 10-day kinetic profile of T cells subjected to fluid shear stress (FSS) ex vivo, with and without stimulation utilizing bead-conjugated anti-CD3/CD28 antibodies. We demonstrate that mechanical stimulation via FSS combined with bead-bound anti-CD3/CD28 antibodies yields a synergistic effect, resulting in amplified and sustained downstream signaling (NF-κB, c-Fos, and NFAT), expression of activation markers (CD69 and CD25), proliferation and production of pro-inflammatory cytokines (IFN-γ, TNF-α, and IL-2). This study represents the first characterization of the dynamic response of primary T cells to FSS. Collectively, our findings underscore the critical role of mechanosensitive ion channel-mediated mechanobiological signaling in T cell activation and fitness, enabling the development of strategies to address the current challenges associated with poor immunotherapy outcomes.
© 2024 The Authors.
-
Homo sapiens (Human)
-
Immunology and Microbiology
Sialic acid-modified der p 2 allergen exerts immunomodulatory effects on human PBMCs.
In The Journal of Allergy and Clinical Immunology. Global on 1 February 2024 by Keumatio Doungtsop, B. C., Nardini, E., et al.
House dust mite extract-based allergen immunotherapy (AIT) to treat house dust mite allergy is substantially effective but still presents some safety and efficacy concerns that warrant improvement. Several major allergen-based approaches to increase safety and efficacy of AIT have been proposed. One of them is the use of the group 2 allergen, Der p 2.
We sought to investigate the immunomodulatory effects of sialic acid-modified major allergen recombinant Der p 2 (sia-rDer p 2) on PBMCs from healthy volunteers.
We activated PBMCs with anti-CD3/CD28 antibodies and incubated them at 37°C for 6 days in the presence or absence of either native rDer p 2 or α2-3 sialic acid-modified rDer p 2 (sia-rDer p 2). We assessed the changes in CD4+ T-cell activation and proliferation by flow cytometry and changes in T-lymphocyte cytokine production in cell culture supernatant by ELISA.
We observed that PBMCs treated with sia-rDer p 2 presented with a markedly decreased expression of CD69 and an increased abundance of LAG-3+ lymphocytes compared with cells treated with rDer p 2. Moreover, PBMCs treated with sia-rDer p 2 showed a reduced production of IL-4, IL-13, and IL-5 and displayed a higher IL-10/IL-5 ratio compared with rDer p 2-treated PBMCs.
We demonstrate that sia-rDer p 2 might be a safer option than native rDer p 2 for Der p 2-specific AIT. This is most relevant in the early phase of AIT that is often characterized by heightened TH2 responses, because sia-rDer p 2 does not enhance the production of TH2 cytokines.
© 2023 The Authors.
-
Homo sapiens (Human)
Bispecific BCMA/CD24 CAR-T cells control multiple myeloma growth.
In Nature Communications on 19 January 2024 by Sun, F., Cheng, Y., et al.
Anti-multiple myeloma B cell maturation antigen (BCMA)-specific chimeric antigen receptor (CAR) T-cell therapies represent a promising treatment strategy with high response rates in myeloma. However, durable cures following anti-BCMA CAR-T cell treatment of myeloma are rare. One potential reason is that a small subset of minimal residual myeloma cells seeds relapse. Residual myeloma cells following BCMA-CAR-T-mediated treatment show less-differentiated features and express stem-like genes, including CD24. CD24-positive myeloma cells represent a large fraction of residual myeloma cells after BCMA-CAR-T therapy. In this work, we develop CD24-CAR-T cells and test their ability to eliminate myeloma cells. We find that CD24-CAR-T cells block the CD24-Siglec-10 pathway, thereby enhancing macrophage phagocytic clearance of myeloma cells. Additionally, CD24-CAR-T cells polarize macrophages to a M1-like phenotype. A dual-targeted BCMA-CD24-CAR-T exhibits improved efficacy compared to monospecific BCMA-CAR-T-cell therapy. This work presents an immunotherapeutic approach that targets myeloma cells and promotes tumor cell clearance by macrophages.
© 2024. The Author(s).
-
FC/FACS
-
Immunology and Microbiology
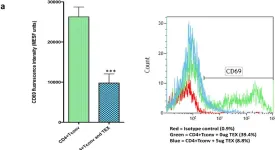
In Sci Rep on 4 February 2016 by Muller, L., Mitsuhashi, M., et al.
Fig.7.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 3
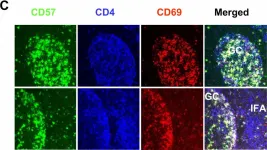
In BMC Immunol on 4 February 2005 by Kim, J. R., Lim, H. W., et al.
Fig.1.C

-
IHC-IF
-
Homo sapiens (Human)
Collected and cropped from BMC Immunol by CiteAb, provided under a CC-BY license
Image 1 of 3
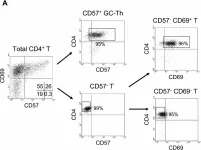
In BMC Immunol on 4 February 2005 by Kim, J. R., Lim, H. W., et al.
Fig.2.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from BMC Immunol by CiteAb, provided under a CC-BY license
Image 1 of 3