Well-defined nanostructures are crucial for precisely understanding nano-bio interactions. However, nanoparticles (NPs) fabricated through conventional synthesis approaches often lack poor controllability and reproducibility. Herein, a synthetic biology-based strategy is introduced to fabricate uniformly reproducible protein-based NPs, achieving precise control over heterogeneous components of the NPs. Specifically, a ferritin assembly toolbox system is developed that enables intracellular assembly of ferritin subunits/variants in Escherichia coli. Using this strategy, a proof-of-concept study is provided to explore the interplay between ligand density of NPs and their tumor targets/penetration. Various ferritin hybrid nanocages (FHn) containing human ferritin heavy chains (FH) and light chains are accurately assembled, leveraging their intrinsic binding with tumor cells and prolonged circulation time in blood, respectively. Further studies reveal that tumor cell uptake is FH density-dependent through active binding with transferrin receptor 1, whereas in vivo tumor accumulation and tissue penetration are found to be correlated to heterogeneous assembly of FHn and vascular permeability of tumors. Densities of 3.7 FH/100 nm2 on the nanoparticle surface exhibit the highest degree of tumor accumulation and penetration, particularly in tumors with high permeability compared to those with low permeability. This study underscores the significance of nanoparticle heterogeneity in determining particle fate in biological systems.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Product Citations: 55
Self-Assembly of Heterogeneous Ferritin Nanocages for Tumor Uptake and Penetration.
In Advanced Science (Weinheim, Baden-Wurttemberg, Germany) on 1 May 2024 by Liu, Q., Wang, C., et al.
-
Cancer Research
Transferrin Receptor Protein 1 Is an Entry Factor for Rabies Virus.
In Journal of Virology on 28 February 2023 by Wang, X., Wen, Z., et al.
Rabies virus (RABV) is a prototypical neurotropic virus that causes rabies in human and animals with an almost 100% mortality rate. Once RABV enters the central nervous system, no treatment is proven to prevent death. RABV glycoprotein (G) interacts with cell surface receptors and then enters cells via clathrin-mediated endocytosis (CME); however, the key host factors involved remain largely unknown. Here, we identified transferrin receptor 1 (TfR1), a classic receptor that undergoes CME, as an entry factor for RABV. TfR1 interacts with RABV G and is involved in the endocytosis of RABV. An antibody against TfR1 or the TfR1 ectodomain soluble protein significantly blocked RABV infection in HEK293 cells, N2a cells, and mouse primary neuronal cells. We further found that the endocytosis of TfR1 is coupled with the endocytosis of RABV and that TfR1 and RABV are transported to early and late endosomes. Our results suggest that RABV hijacks the transport pathway of TfR1 for entry, thereby deepening our understanding of the entry mechanism of RABV. IMPORTANCE For most viruses, cell entry involves engagement with many distinct plasma membrane components, each of which is essential. After binding to its specific receptor(s), rabies virus (RABV) enters host cells through the process of clathrin-mediated endocytosis. However, whether the receptor-dependent clathrin-mediated endocytosis of RABV requires other plasma membrane components remain largely unknown. Here, we demonstrate that transferrin receptor 1 (TfR1) is a functional entry factor for RABV infection. The endocytosis of RABV is coupled with the endocytosis of TfR1. Our results indicate that RABV hijacks the transport pathway of TfR1 for entry, which deepens our understanding of the entry mechanism of RABV.
-
Immunology and Microbiology
In Journal of Virology on 28 February 2023 by Wang, X., Wen, Z., et al.
Identification of bona fide functional receptors and elucidation of the mechanism of receptor-mediated virus entry are important to reveal targets for developing therapeutics against rabies virus (RABV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Our previous studies suggest that metabotropic glutamate receptor subtype 2 (mGluR2) functions as an entry receptor for RABV in vitro, and is an important internalization factor for SARS-CoV-2 in vitro and in vivo. Here, we demonstrate that mGluR2 facilitates RABV internalization in vitro and infection in vivo. We found that transferrin receptor 1 (TfR1) interacts with mGluR2 and internalizes with mGluR2 and RABV in the same clathrin-coated pit. Knockdown of TfR1 blocks agonist-triggered internalization of mGluR2. Importantly, TfR1 also interacts with the SARS-CoV-2 spike protein and is important for SARS-CoV-2 internalization. Our findings identify a novel axis (mGluR2-TfR1 axis) used by RABV and SARS-CoV-2 for entry, and reveal TfR1 as a potential target for therapeutics against RABV and SARS-CoV-2. IMPORTANCE We previously found that metabotropic glutamate receptor subtype 2 (mGluR2) is an entry receptor for RABV in vitro, and an important internalization factor for SARS-CoV-2 in vitro and in vivo. However, whether mGluR2 is required for RABV infection in vivo was unknown. In addition, how mGluR2 mediates the internalization of RABV and SARS-CoV-2 needed to be resolved. Here, we found that mGluR2 gene knockout mice survived a lethal challenge with RABV. To our knowledge, mGluR2 is the first host factor to be definitively shown to play an important role in RABV street virus infection in vivo. We further found that transferrin receptor protein 1 (TfR1) directly interacts and cooperates with mGluR2 to regulate the endocytosis of RABV and SARS-CoV-2. Our study identifies a novel axis (mGluR2-TfR1 axis) used by RABV and SARS-CoV-2 for entry and opens a new door for the development of therapeutics against RABV and SARS-CoV-2.
-
ICC-IF
-
COVID-19
-
Immunology and Microbiology
Spatial regulation of the glycocalyx component podocalyxin is a switch for prometastatic function.
In Science Advances on 3 February 2023 by Román-Fernández, Á., Mansour, M. A., et al.
The glycocalyx component and sialomucin podocalyxin (PODXL) is required for normal tissue development by promoting apical membranes to form between cells, triggering lumen formation. Elevated PODXL expression is also associated with metastasis and poor clinical outcome in multiple tumor types. How PODXL presents this duality in effect remains unknown. We identify an unexpected function of PODXL as a decoy receptor for galectin-3 (GAL3), whereby the PODXL-GAL3 interaction releases GAL3 repression of integrin-based invasion. Differential cortical targeting of PODXL, regulated by ubiquitination, is the molecular mechanism controlling alternate fates. Both PODXL high and low surface levels occur in parallel subpopulations within cancer cells. Orthotopic intraprostatic xenograft of PODXL-manipulated cells or those with different surface levels of PODXL define that this axis controls metastasis in vivo. Clinically, interplay between PODXL-GAL3 stratifies prostate cancer patients with poor outcome. Our studies define the molecular mechanisms and context in which PODXL promotes invasion and metastasis.
Spatial regulation of the glycocalyx component Podocalyxin is a switch for pro-metastatic function
Preprint on BioRxiv : the Preprint Server for Biology on 4 November 2022 by Román-Fernández, Á., Mansour, M. A., et al.
The glycocalyx component and sialomucin Podocalyxin (PODXL) is required for normal tissue development by promoting apical membranes to form between cells, triggering lumen formation. Elevated PODXL expression is also associated with metastasis and poor clinical outcome in multiple tumour types. How PODXL presents this duality in effect remains unknown. We identify an unexpected function of PODXL as a decoy receptor for Galectin-3 (GAL3), whereby the PODXL-GAL3 interaction releases GAL3 repression of integrin-based invasion. Differential cortical targeting of PODXL, regulated by ubiquitination, is the molecular mechanism controlling alternate fates. Both PODXL high versus low surface levels occur in parallel subpopulations within cancer cells. Orthotopic intraprostatic xenograft of PODXL-manipulated cells or those with different surface levels of PODXL define that this axis controls metastasis in vivo . Clinically, interplay between PODXL-GAL3 stratifies prostate cancer patients with poor outcome. Our studies define the molecular mechanisms and context in which PODXL promotes invasion and metastasis.
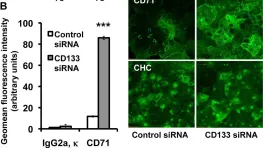
In PLoS One on 4 October 2011 by Bourseau-Guilmain, E., Griveau, A., et al.
Fig.4.B

-
ICC-IF
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 1