Hepatocellular carcinoma (HCC) resists immunotherapy due to its immunosuppressive microenvironment. Sarcoma homology 2 domain-containing protein tyrosine phosphatase-1 (SHP-1) inhibits T cell receptor signaling, and its pharmacological inhibition is limited by poor selectivity and membrane permeability. Here, we generated CRISPR-edited SHP-1-knockout (KO) CD8+ T cells to enhance adoptive therapy against HCC. Single-cell RNA sequencing of HCC patient T cells revealed elevated SHP-1 in exhausted subsets. SHP-1-KO T cells exhibited increased effector memory T cells (TEM) proportions and enhanced IFN-γ/Granzyme B/perforin secretion, improving cytotoxicity against HCC lines. In humanized PDX models, SHP-1-KO T cells demonstrated superior tumor-killing activity. Transcriptomics identified upregulated lipid metabolism pathways, with HMGCR as a hub gene. Combining SHP-1-KO T cells with simvastatin (HMGCR inhibitor) synergistically amplified anti-HCC efficacy. This study proposes a dual strategy combining SHP-1-targeted cell therapy and metabolic modulation to overcome immunotherapy resistance, offering a translatable approach for HCC treatment.
© 2025 The Author(s).
Product Citations: 43
In IScience on 18 April 2025 by Liu, H., Ge, W., et al.
-
Cancer Research
IL-7 promotes CD19-directed CAR-T cells proliferation through miRNA-98-5p by targeting CDKN1A.
In International Immunopharmacology on 1 November 2023 by Yang, L. R., Li, L., et al.
CAR-T targeting CD19 have achieved significant effects in the treatment of B-line leukemia and lymphoma. However, the treated patients frequently relapsed and could not achieve complete remission. Therefore, improving the proliferation and cytotoxicity of CAR-T cells, reducing exhaustion and enhancing infiltration capacity are still issues to be solved. The IL-7 has been shown to enhance the memory characteristics of CAR-T cells, but the specific mechanism has yet to be elaborated. miRNAs play an important role in T cell activity. However, whether miRNA is involved in the activation of CAR-T cells by IL-7 has not yet been reported. Our previous study had established the 3rd generation CAR-T cells. The present study further found that IL-7 significantly increased the proliferation of anti-CD19 CAR-T cells, the ratio of CD4 + CAR + cells and the S phase of cell cycle. In vivo study NAMALWA xenograft model showed that IL-7-stimulated CAR-T cells possessed stronger tumoricidal efficiency. Further we validated that IL-7 induced CAR-T cells had low expression of CDKN1A and high expression of miRNA-98-5p. Additionally, CDKN1A was associated with miRNA-98-5p. Our results, for the first time, suggested IL-7 could conspicuously enhance the proliferation of CAR-T cells through miRNA-98-5p targeting CDKN1A expression, which should be applied to CAR-T production.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
-
FC/FACS
-
Genetics
-
Immunology and Microbiology
In Cancer Res Commun on 1 May 2023 by Islam, S. M. R., Maeda, T., et al.
Tumor-infiltrating lymphocytes (TIL) that can recognize and kill tumor cells have curative potential in subsets of patients treated with adoptive cell transfer (ACT). However, lack of TIL therapeutic efficacy in many patients may be due in large part to a paucity of tumor-reactive T cells in TIL and the exhausted and terminally differentiated status of those tumor-reactive T cells. We sought to reprogram exhausted TIL that possess T-cell receptors (TCR) specific for tumor antigens into induced pluripotent stem cells (iPSC) to rejuvenate them for more potent ACT. We first attempted to reprogram tumor neoantigen-specific TIL by αCD3 Ab prestimulation which resulted in failure of establishing tumor-reactive TIL-iPSCs, instead, T cell-derived iPSCs from bystander T cells were established. To selectively activate and enrich tumor-reactive T cells from the heterogenous TIL population, CD8+ PD-1+ 4-1BB+ TIL population were isolated after coculture with autologous tumor cells, followed by direct reprogramming into iPSCs. TCR sequencing analysis of the resulting iPSC clones revealed that reprogrammed TIL-iPSCs encoded TCRs that were identical to the pre-identified tumor-reactive TCRs found in minimally cultured TIL. Moreover, reprogrammed TIL-iPSCs contained rare tumor antigen-specific TCRs, which were not detectable by TCR sequencing of the starting cell population. Thus, reprogramming of PD-1+ 4-1BB+ TIL after coculture with autologous tumor cells selectively generates tumor antigen-specific TIL-iPSCs, and is a distinctive method to enrich and identify tumor antigen-specific TCRs of low frequency from TIL.
Reprogramming of TIL into iPSC holds great promise for the future treatment of cancer due to their rejuvenated nature and the retention of tumor-specific TCRs. One limitation is the lack of selective and efficient methods for reprogramming tumor-specific T cells from polyclonal TIL. Here we addressed this limitation and present a method to efficiently reprogram TIL into iPSC colonies carrying diverse tumor antigen reactive TCR recombination.
© 2023 The Authors; Published by the American Association for Cancer Research.
-
Cancer Research
-
Stem Cells and Developmental Biology
IL-7 promotes CD19-directed CAR-T cells proliferation through miRNA-98-5p by targeting CDKN1A
Preprint on Research Square on 6 March 2023 by Hou, Z., Yang, L., et al.
CAR-T targeting CD19 have achieved significant effects in the treatment of B-line leukemia and lymphoma. However, the treated patients frequently relapsed and could not achieve complete remission. Therefore, improving the proliferation and cytotoxicity of CAR-T cells, reducing exhaustion and enhancing infiltration capacity are still issues to be solved. The IL-7 has been shown to enhance the memory characteristics of CAR-T cells, but the specific mechanism has yet to be elaborated. miRNAs play an important role in T cell activity. However, whether miRNA is involved in the activation of CAR-T cells by IL-7 has not yet been reported. Our previous study had established the 3rd generation CAR-T cells. The present study further found that IL-7 significantly increased the proliferation of anti-CD19 CAR-T cells, the ratio of CD4 + CAR + cells and the S phase of cell cycle. In vivo study showed that IL-7-stimulated CAR-T possessed stronger tumoricidal efficiency. Further we validated that IL-7 induced CAR-T cells had low expression of CDKN1A and high expression of miRNA-98-5p. Additionally, CDKN1A was associated with miRNA-98-5p. Our results, for the first time, suggested IL-7 could conspicuously enhance the proliferation of CAR-T cells through miRNA-98-5p targeting CDKN1A expression, which should be applied to CAR-T production.
-
FC/FACS
-
Genetics
-
Immunology and Microbiology
In Cell Transplantation on 17 January 2023 by Cao, L., Ma, X., et al.
Clinically, xenotransplantation often leads to T-cell-mediated graft rejection. Immunosuppressive agents including polyclonal regulatory T cells (poly-Tregs) promote global immunosuppression, resulting in serious infections and malignancies in patients. Xenoantigen-expanded Tregs (xeno-Tregs) have become a promising immune therapy strategy to protect xenografts with fewer side effects. In this study, we aimed to identify an efficient and stable subset of xeno-Tregs. We enriched CD27+ xeno-Tregs using cell sorting and evaluated their suppressive functions and stability in vitro via mixed lymphocyte reaction (MLR), real-time polymerase chain reaction, inflammatory induction assay, and Western blotting. A STAT5 inhibitor was used to investigate the relationship between the function and stability of CD27+ xeno-Tregs and the JAK3-STAT5 signaling pathway. A humanized xenotransplanted mouse model was used to evaluate the function of CD27+ xeno-Tregs in vivo. Our results show that CD27+ xeno-Tregs express higher levels of Foxp3, cytotoxic T-lymphocyte antigen-4 (CTLA4), and Helios and lower levels of interleukin-17 (IL-17) than their CD27- counterparts. In addition, CD27+ xeno-Tregs showed enhanced suppressive function in xeno-MLR at ratios of 1:4 and 1:16 of Tregs:responder cells. Under inflammatory conditions, a lower percentage of CD27+ xeno-Tregs secretes IL-17 and interferon-γ (IFN-γ). CD27+ xeno-Tregs demonstrated an upregulated JAK3-STAT5 pathway compared with that of CD27- xeno-Tregs and showed decreased Foxp3, Helios, and CTLA4 expression after addition of STAT5 inhibitor. Mice that received porcine skin grafts showed a normal tissue phenotype and less leukocyte infiltration after reconstitution with CD27+ xeno-Tregs. Taken together, these data indicate that CD27+ xeno-Tregs may suppress immune responses in a xenoantigen-specific manner, which might be related to the activation of the JAK3-STAT5 signaling pathway.
-
FC/FACS
-
Immunology and Microbiology
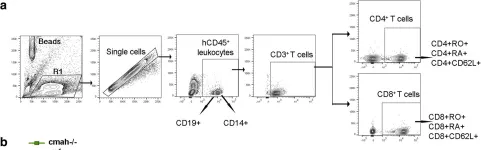
In BMC Immunol on 7 January 2019 by Dagur, R. S., Branch-Woods, A., et al.
Fig.7.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from BMC Immunol by CiteAb, provided under a CC-BY license
Image 1 of 2
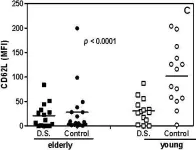
In Immun Ageing on 28 March 2009 by Gasparoto, T. H., Vieira, N. A., et al.
Fig.2.C

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Immun Ageing by CiteAb, provided under a CC-BY license
Image 1 of 2