We describe humans with rare biallelic loss-of-function PTCRA variants impairing pre-α T cell receptor (pre-TCRα) expression. Low circulating naive αβ T cell counts at birth persisted over time, with normal memory αβ and high γδ T cell counts. Their TCRα repertoire was biased, which suggests that noncanonical thymic differentiation pathways can rescue αβ T cell development. Only a minority of these individuals were sick, with infection, lymphoproliferation, and/or autoimmunity. We also report that 1 in 4000 individuals from the Middle East and South Asia are homozygous for a common hypomorphic PTCRA variant. They had normal circulating naive αβ T cell counts but high γδ T cell counts. Although residual pre-TCRα expression drove the differentiation of more αβ T cells, autoimmune conditions were more frequent in these patients compared with the general population.
Product Citations: 17
The immunopathological landscape of human pre-TCRα deficiency: From rare to common variants.
In Science on 1 March 2024 by Materna, M., Delmonte, O. M., et al.
In Viruses on 13 May 2022 by Sabino, J. S., Amorim, M. R., et al.
Currently, there are no evidence-based treatment options for long COVID-19, and it is known that SARS-CoV-2 can persist in part of the infected patients, especially those with immunosuppression. Since there is a robust secretion of SARS-CoV-2-specific highly-neutralizing IgA antibodies in breast milk, and because this immunoglobulin plays an essential role against respiratory virus infection in mucosa cells, being, in addition, more potent in neutralizing SARS-CoV-2 than IgG, here we report the clinical course of an NFκB-deficient patient chronically infected with the SARS-CoV-2 Gamma variant, who, after a non-full effective treatment with plasma infusion, received breast milk from a vaccinated mother by oral route as treatment for COVID-19. After such treatment, the symptoms improved, and the patient was systematically tested negative for SARS-CoV-2. Thus, we hypothesize that IgA and IgG secreted antibodies present in breast milk could be useful to treat persistent SARS-CoV-2 infection in immunodeficient patients.
-
FC/FACS
-
COVID-19
-
Genetics
Microbial exposure during early human development primes fetal immune cells.
In Cell on 24 June 2021 by Mishra, A., Lai, G. C., et al.
The human fetal immune system begins to develop early during gestation; however, factors responsible for fetal immune-priming remain elusive. We explored potential exposure to microbial agents in utero and their contribution toward activation of memory T cells in fetal tissues. We profiled microbes across fetal organs using 16S rRNA gene sequencing and detected low but consistent microbial signal in fetal gut, skin, placenta, and lungs in the 2nd trimester of gestation. We identified several live bacterial strains including Staphylococcus and Lactobacillus in fetal tissues, which induced in vitro activation of memory T cells in fetal mesenteric lymph node, supporting the role of microbial exposure in fetal immune-priming. Finally, using SEM and RNA-ISH, we visualized discrete localization of bacteria-like structures and eubacterial-RNA within 14th weeks fetal gut lumen. These findings indicate selective presence of live microbes in fetal organs during the 2nd trimester of gestation and have broader implications toward the establishment of immune competency and priming before birth.
Crown Copyright © 2021. Published by Elsevier Inc. All rights reserved.
-
Immunology and Microbiology
A Single-Cell Immune Atlas of Triple Negative Breast Cancer Reveals Novel Immune Cell Subsets
Preprint on Research Square on 2 September 2020 by Qiu, S., Hong, R., et al.
h4>Background: /h4> Triple-negative breast cancer (TNBC) represents the most aggressive breast cancer subtype, which recently attracts great interest for immune therapeutic development. In this context, in-depth understanding of TNBC immune landscape is highly demanded. h4>Results: /h4>: Here we report full-length single-cell RNA sequencing results of 9683 tumor-infiltrated immune cells isolated from 14 treatment naïve TNBC tumors, where 22 immune cell subsets, including T cells, macrophages, B cells, and DCs have been characterized. We identify a new T cell subset, CD8 + CXCL8 + T cell, which associates with poor survival, and a subset of “pre-exhaustion” T cell cluster, which is predictive of favorable prognosis. A novel immune cell subset comprised of TCR + macrophages, is found to be widely distributed in TNBC tumors. Further analyses reveal an up-regulation of molecules associated with TCR signaling and cytotoxicity in these immune cells. h4>Conclusions: /h4>: Altogether, our study provides a valuable resource to understand the immune ecosystem of TNBC. The novel immune cell subsets reported herein might be functionally important in cancer immunity. These data will be helpful for the immunotherapeutic strategy design of this disease.
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
Generation of T cells from Human and Nonhuman Primate Pluripotent Stem Cells.
In Bio-protocol on 5 July 2020 by Kumar, A., D'Souza, S. S., et al.
Pluripotent stem cells (PSCs) have the potential to provide homogeneous cell populations of T cells that can be grown at a clinical scale and genetically engineered to meet specific clinical needs. OP9-DLL4, a stromal line ectopically expressing the Notch ligand Delta-like 4 (DLL4) is used to support differentiation of PSCs to T-lymphocytes. This article outlines several protocols related to generation of T cells from human and non-human primate (NHP) PSCs, including initial hematopoietic differentiation of PSC on OP9 feeders or defined conditions, followed by coculture of the OP9-DLL4 cells with the PSC-derived hematopoietic progenitors (HPs), leading to efficient differentiation to T lymphocytes. In addition, we describe a protocol for robust T cell generation from hPSCs conditionally expressing ETS1. The presented protocols provide a platform for T cell production for disease modeling and evaluating their use for immunotherapy in large animal models.
Copyright © 2020 The Authors; exclusive licensee Bio-protocol LLC.
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
In Front Immunol on 25 February 2015 by Ziegler, H., Welker, C., et al.
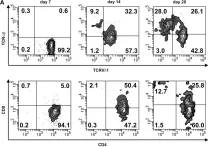
Fig.5.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 2
In Front Immunol on 25 February 2015 by Ziegler, H., Welker, C., et al.
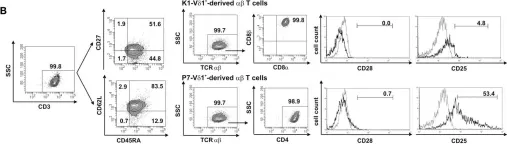
Fig.5.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 2