This study aimed to investigate the therapeutic effect of human nasal turbinate-derived stem cells (hNTSCs) on mice with rheumatoid arthritis (RA) and identify hNTSC gene signatures with therapeutic effects on RA. hNTSCs were obtained from 20 healthy controls (HCs) who had undergone nasal turbinate surgery. Collagen-induced arthritis (CIA) mice were used to investigate the therapeutic effects of hNTSCs. The engraftment and migration abilities of hNTSCs were evaluated. CD4+CD25- T cells were co-cultured with hNTSCs, and effector T cell proliferation was evaluated by flow cytometry. Osteoclast differentiation was evaluated using mouse bone marrow monocytes which were cultured with M-CSF and RANKL, then TRAP staining was performed to measure effect of hNTSCs on osteoclastogenesis. Microarray assays were performed to identify gene expression differences between hNTSCs with CIA mice therapeutic or not and were validated by RT-qPCR. hNTSCs differentiated well into osteoblasts and adipocytes and expressed high levels of CXCL1 and osteoprotegerin. Single-cell RNA sequencing showed that hNTSCs clustered into 11 cell types, and cell surface markers were compatible with mesenchymal stem cells. hNTSC-treated CIA mice showed reductions in arthritis severity scores and incidence of arthritis. In engraft measurements, hNTSCs survived for 8 to 12 weeks in mice paws. Chemokine receptors expression increased in hNTSCs by IL-1β or TNF-α stimulation. CD4+CD25- T cell proliferation was reduced by hNTSCs and reversed by adding 1-MT (indoleamine 2,3-dioxygenase inhibitor), indicating that indoleamine 2,3-dioxygenase mediated T cell suppression. Osteoclastogenesis was suppressed by hNTSCs, and this was attenuated by anti-OPG Ab. hNTSCs therapeutic in CIA mice showed specific gene signatures with up-regulated genes (KRTAP1-5, HAS2, and CXCL1) and down-regulated genes (GSTT2B and C4B) compared to hNTSCs without CIA therapeutic effects. hNTSCs exhibited therapeutic potential in RA. Therapeutic effects were mediated by effector helper T cell suppression and the inhibition of osteoclastogenesis. In addition, hNTSCs with greater therapeutic effects on RA showed significant differences in their gene signatures.
© 2025. The Author(s).
Product Citations: 177
In Scientific Reports on 22 February 2025 by Lee, J., Min, H. K., et al.
-
ICC-IF
-
FC/FACS
-
Homo sapiens (Human)
-
Stem Cells and Developmental Biology
In Journal of Translational Medicine on 18 November 2024 by Sun, H., Zhai, H., et al.
Mesenchymal stem cells (MSCs) have been proposed to treat osteoarthritis (OA) for many years. However, clinical outcomes have been inconsistent due to biological variation between patients, differences in tissue source and preparation of the MSCs, and type of donor (e.g. allogenic versus autologous). Here, we test the hypothesis that inconsistent clinical outcomes are related to variations in the stemness and senescence of the injected autologous adipose-derived (AD) MSCs.
In the prospective randomized trial, 45 knee OA patients were divided into two groups: Group 1 (n = 22) patients treated with high tibial osteotomy (HTO) alone and Group 2 (n = 23) patients treated with HTO followed by intra-articular injection of autologous AD-MSCs (HTO + AD-MSCs). MRI and X-ray were performed pre-operation and 12 months post-operation. WOMAC and VAS score were collected four times, every 6 months over a 24-month follow-up. We observed the proliferation and stemness of AD-MSCs selected from the 5 patients showing the most improvement and from the 5 patients with the least improvement, and completed further in vitro experiments including beta-galactosidase activity, reactive oxygen species and bioinformatic analysis.
The results showed that patients treated with HTO + AD-MSCs had a significant reduction in knee OA severity as compared to patients treated with HTO alone. Moreover, we discovered that proliferation and colony forming efficiency of AD-MSCs selected from the 5 patients showing the most improvement performed significantly better than cells selected from the 5 patients with the least improvement. AD-MSCs from the patients with the most improvement also had lower amounts of senescent cells and intracellular reactive oxygen species.
Clinical outcomes of autologous AD-MSCs therapy in knee osteoarthritis are correlated with stem cell stemness and senescence. Our study highlights emerging opportunities and trends in precision medicine that could potentially improve autologous MSC-based therapies.
© 2024. The Author(s).
-
Stem Cells and Developmental Biology
In Cell Stem Cell on 1 August 2024 by Jakobsen, N. A., Turkalj, S., et al.
Clonal hematopoiesis (CH) arises when hematopoietic stem cells (HSCs) acquire mutations, most frequently in the DNMT3A and TET2 genes, conferring a competitive advantage through mechanisms that remain unclear. To gain insight into how CH mutations enable gradual clonal expansion, we used single-cell multi-omics with high-fidelity genotyping on human CH bone marrow (BM) samples. Most of the selective advantage of mutant cells occurs within HSCs. DNMT3A- and TET2-mutant clones expand further in early progenitors, while TET2 mutations accelerate myeloid maturation in a dose-dependent manner. Unexpectedly, both mutant and non-mutant HSCs from CH samples are enriched for inflammatory and aging transcriptomic signatures, compared with HSCs from non-CH samples, revealing a non-cell-autonomous effect. However, DNMT3A- and TET2-mutant HSCs have an attenuated inflammatory response relative to wild-type HSCs within the same sample. Our data support a model whereby CH clones are gradually selected because they are resistant to the deleterious impact of inflammation and aging.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
In Stem Cells Translational Medicine on 15 July 2024 by Tesch, R. S., Takamori, E. R., et al.
Condylar resorption is an aggressive and disability form of temporomandibular joint (TMJ) degenerative disease, usually non-respondent to conservative or minimally invasive therapies and often leading to surgical intervention and prostheses implantation. This condition is also one of the most dreaded postoperative complications of orthognathic surgery, with severe cartilage erosion and loss of subchondral bone volume and mineral density, associated with a painful or not inflammatory processes. Because regenerative medicine has emerged as an alternative for orthopedic cases with advanced degenerative joint disease, we conducted a phase I/IIa clinical trial (U1111-1194-6997) to evaluate the safety and efficacy of autologous nasal septal chondroprogenitor cells. Ten participants underwent biopsy of the nasal septum cartilage during their orthognathic surgery. The harvested cells were cultured in vitro and analyzed for viability, presence of phenotype markers for mesenchymal stem and/or chondroprogenitor cells, and the potential to differentiate into chondrocytes, adipocytes, and osteoblasts. After the intra-articular injection of the cell therapy, clinical follow-up was performed using the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) and computed tomography (CT) images. No serious adverse events related to the cell therapy injection were observed during the 12-month follow-up period. It was found that autologous chondroprogenitors reduced arthralgia, promoted stabilization of mandibular function and condylar volume, and regeneration of condylar tissues. This study demonstrates that chondroprogenitor cells from the nasal septum may be a promise strategy for the treatment of temporomandibular degenerative joint disease that do not respond to other conservative therapies.
© The Author(s) 2024. Published by Oxford University Press.
-
Stem Cells and Developmental Biology
In Cellular and Molecular Life Sciences : CMLS on 11 July 2024 by Kushida, Y., Oguma, Y., et al.
Muse cells, identified as cells positive for the pluripotent surface marker SSEA-3, are pluripotent-like endogenous stem cells located in the bone marrow (BM), peripheral blood, and organ connective tissues. The detailed characteristics of SSEA-3(+) cells in extraembryonic tissue, however, are unknown. Here, we demonstrated that similar to human-adult tissue-Muse cells collected from the BM, adipose tissue, and dermis as SSEA-3(+), human-umbilical cord (UC)-SSEA-3(+) cells express pluripotency markers, differentiate into triploblastic-lineage cells at a single cell level, migrate to damaged tissue, and exhibit low telomerase activity and non-tumorigenicity. Notably, ~ 20% of human-UC-SSEA-3(+) cells were negative for X-inactive specific transcript (XIST), a naïve pluripotent stem cell characteristic, whereas all human adult tissue-Muse cells are XIST-positive. Single-cell RNA sequencing revealed that the gene expression profile of human-UC-SSEA-3(+) cells was more similar to that of human post-implantation blastocysts than human-adult tissue-Muse cells. The DNA methylation level showed the same trend, and notably, the methylation levels in genes particularly related to differentiation were lower in human-UC-SSEA-3(+) cells than in human-adult tissue-Muse cells. Furthermore, human-UC-SSEA-3(+) cells newly express markers specific to extraembryonic-, germline-, and hematopoietic-lineages after differentiation induction in vitro whereas human-adult tissue-Muse cells respond only partially to the induction. Among various stem/progenitor cells in living bodies, those that exhibit properties similar to post-implantation blastocysts in a naïve state have not yet been found in humans. Easily accessible human-UC-SSEA-3(+) cells may be a valuable tool for studying early-stage human development and human reproductive medicine.
© 2024. The Author(s).
-
Biochemistry and Molecular biology
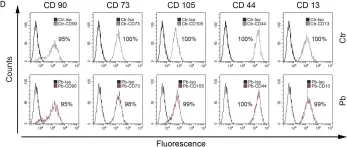
In PLoS One on 27 December 2018 by Griukova, A., Deryabin, P., et al.
Fig.1.D

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3
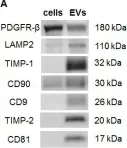
In Oncotarget on 10 March 2015 by Vallabhaneni, K. C., Penfornis, P., et al.
Fig.2.A

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 3
In Stem Cell Res Ther on 4 December 2014 by Wang, Y., Wu, H., et al.
Fig.2.D
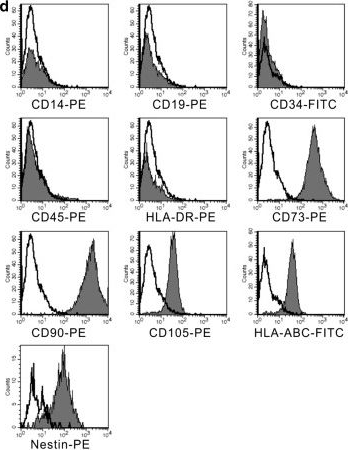
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Stem Cell Res Ther by CiteAb, provided under a CC-BY license
Image 1 of 3