Limited information exists regarding the impact of interferons (IFNs) on the information carried by extracellular vesicles (EVs). This study aimed at investigating whether IFN-α2b, IFN-β, IFN-γ, and IFN-λ1/2 modulate the content of EVs released by primary monocyte-derived macrophages (MDM). Small-EVs (sEVs) were purified by size exclusion chromatography from supernatants of MDM treated with IFNs. To characterize the concentration and dimensions of vesicles, nanoparticle tracking analysis was used. SEVs surface markers were examined by flow cytometry. IFN treatments induced a significant down-regulation of the exosomal markers CD9, CD63, and CD81 on sEVs, and a significant modulation of some adhesion molecules, major histocompatibility complexes and pro-coagulant proteins, suggesting IFNs influence biogenesis and shape the immunological asset of sEVs. SEVs released by IFN-stimulated MDM also impact lymphocyte function, showing significant modulation of lymphocyte activation and IL-17 release. Altogether, our results show that sEVs composition and activity are affected by IFN treatment of MDM.
© 2024 The Authors.
Product Citations: 24
In IScience on 21 June 2024 by Giannessi, F., Percario, Z., et al.
-
Homo sapiens (Human)
In Polymers on 29 September 2023 by Pérez-Díaz, M. A., Martínez-Colin, E. J., et al.
Cross-linked polymer blends from natural compounds, namely gelatin (Gel), chitosan (CS), and synthetic poly (vinyl alcohol) (PVA), have received increasing scrutiny because of their versatility, biocompatibility, and ease of use for tissue engineering. Previously, Gel/CS/PVA [1:1:1] hydrogel produced via the freeze-drying process presented enhanced mechanical properties. This study aimed to investigate the biocompatibility and chondrogenic potential of a steam-sterilized Gel/CS/PVA hydrogel using differentiation of human adipose-derived mesenchymal stromal cells (AD-hMSC) and cartilage marker expression. AD-hMSC displayed fibroblast-like morphology, 90% viability, and 69% proliferative potential. Mesenchymal profiles CD73 (98.3%), CD90 (98.6%), CD105 (97.0%), CD34 (1.11%), CD45 (0.27%), HLA-DR (0.24%); as well as multilineage potential, were confirmed. Chondrogenic differentiation of AD-hMSC in monolayer revealed the formation of cartilaginous nodules composed of glycosaminoglycans after 21 days. Compared to nonstimulated cells, hMSC-derived chondrocytes shifted the expression of CD49a from 2.82% to 40.6%, CD49e from 51.4% to 92.2%, CD54 from 9.66 to 37.2%, and CD151 from 45.1% to 75.8%. When cultured onto Gel/CS/PVA hydrogel during chondrogenic stimulation, AD-hMSC changed to polygonal morphology, and chondrogenic nodules increased by day 15, six days earlier than monolayer-differentiated cells. SEM analysis showed that hMSC-derived chondrocytes adhered to the surface with extended filopodia and abundant ECM formation. Chondrogenic nodules were positive for aggrecan and type II collagen, two of the most abundant components in cartilage. This study supports the biocompatibility of AD-hMSC onto steam-sterilized GE/CS/PVA hydrogels and its improved potential for chondrocyte differentiation. Hydrogel properties were not altered after steam sterilization, which is relevant for biosafety and biomedical purposes.
-
FC/FACS
In STAR Protocols on 17 December 2021 by Houtsma, R., Hogeling, S. M., et al.
Many cancers, including leukemias, are dynamic oligoclonal diseases. Tools to identify and prospectively isolate genetically distinct clones for functional studies are needed. We describe our CombiFlow protocol, which is a combinatorial flow cytometry-based approach to identify and isolate such distinct clones. CombiFlow enables the visualization of clonal evolution during disease progression and the identification of potential relapse-inducing cells at minimal residual disease (MRD) time points. The protocol can be adapted to various research questions and allows functional studies on live sorted cell populations. For complete details on the use and execution of this protocol, please refer to de Boer et al. (2018).
© 2021 The Author(s).
Preprint on Research Square on 29 September 2021 by Verma, Y. K., Yadav, N., et al.
h4>Background: /h4> The interaction of integrins and growth factor receptors in cells is tightly regulated, ensuring cell survival, proliferation, differentiation, adhesion, and migration. The IR generates reactive oxygen species, which leads to ECM remodeling and cell adhesion through the activation of proteases, soluble cytokines and growth factors. Integrins and adhesion of cells to ECM confer higher resistance to ionizing radiation and cytotoxic drug, a phenomenon known as cell adhesion mediated radiation resistance (CAM-RR) and cell adhesion mediated drug resistance (CAM-DR) . Integrins’ involvement in CML progression is well appreciated through its survival, adhesion and migration signaling. The evaluation of global genetic response (in microarray) of ionizing radiation (IR) on integrins expression has not been attempted to specify theirrole and other cell adhesion molecules (CAMs) in CML. In this study, we have compared the microarray based CAMs response in myelogenous leukemia cells onIR exposure. h4>Results: /h4>: Results revealed differential regulation of many CAMs, with strongest expression of integrin β2 (CD18), whose role has not been fully appreciated in CML due to low level of expression. However, the synergistic LiCl(GSK3β inhibitor) and IR treatment significantly upregulates CD18 expression leading to enhanced survival, cell adhesion mediated drug/radiation resistance (CAM-DR/RR) and transcription of migration related genes. These effects could be undermined in the presence of CD18 antibody. This may be one of the reasons for CML resistance to radiation therapy and its relapse upon stem cell transplantation. h4>Conclusion: /h4> This study proposes CD18 antagonist administration as an adjuvant in anti-CML therapy and other cancers in which it displays aberrant expression subsuming the contraindication of GSK3β inhibitor. Nevertheless, the CD18 mediated cell adhesion in tumor progression beckons development of improved drug regimensandidentification of diagnostic and prognostic signature for CML.
-
FC/FACS
-
Homo sapiens (Human)
-
Genetics
In Journal of Medicinal Chemistry on 27 May 2021 by Tomassi, S., D'Amore, V. M., et al.
Over recent years, αvβ6 and αvβ8 Arg-Gly-Asp (RGD) integrins have risen to prominence as interchangeable co-receptors for the cellular entry of herpes simplex virus-1 (HSV-1). In fact, the employment of subtype-specific integrin-neutralizing antibodies or gene-silencing siRNAs has emerged as a valuable strategy for impairing HSV infectivity. Here, we shift the focus to a more affordable pharmaceutical approach based on small RGD-containing cyclic pentapeptides. Starting from our recently developed αvβ6-preferential peptide [RGD-Chg-E]-CONH2 (1), a small library of N-methylated derivatives (2-6) was indeed synthesized in the attempt to increase its affinity toward αvβ8. Among the novel compounds, [RGD-Chg-(NMe)E]-CONH2 (6) turned out to be a potent αvβ6/αvβ8 binder and a promising inhibitor of HSV entry through an integrin-dependent mechanism. Furthermore, the renewed selectivity profile of 6 was fully rationalized by a NMR/molecular modeling combined approach, providing novel valuable hints for the design of RGD integrin ligands with the desired specificity profile.
-
Homo sapiens (Human)
-
Chemistry
-
Immunology and Microbiology
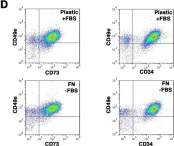
In Sci Rep on 14 March 2017 by Di Maggio, N., Martella, E., et al.
Fig.5.D
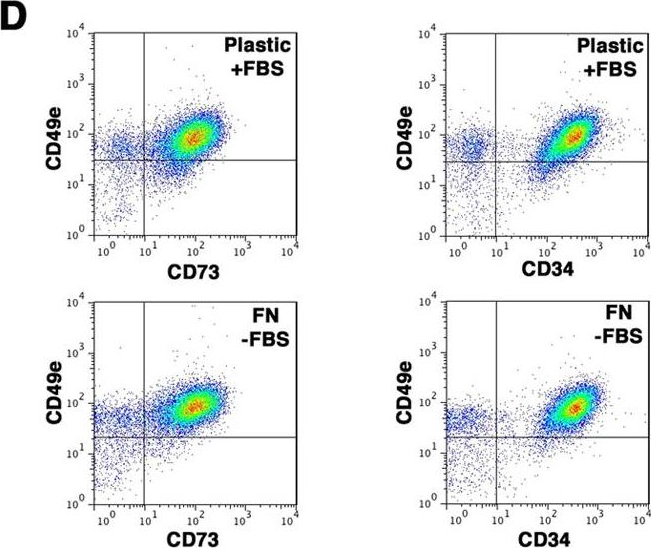
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 2
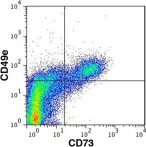
In Sci Rep on 14 March 2017 by Di Maggio, N., Martella, E., et al.
Fig.7.C

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 2