Nanrilkefusp alfa (nanril; SOT101) is an interleukin (IL)-15 receptor βγ superagonist that stimulates natural killer (NK) and CD8+ T cells, thereby promoting an innate and adaptive anti-tumor inflammatory microenvironment in mouse tumor models either in monotherapy or combined with an anti-programmed cell death protein 1 (PD-1) antibody. In cynomolgus monkeys, a clinical schedule was identified, which translated into the design of a phase 1/1b clinical trial, AURELIO-03 (NCT04234113). In 51 patients with advanced/metastatic solid tumors, nanril increased the proportions of CD8+ T cells and NK cells in peripheral blood and tumors. It had a favorable safety profile when administered subcutaneously on days 1, 2, 8, and 9 of each 21-day cycle as monotherapy (0.25-15 μg/kg) or combined (1.5-12 μg/kg) with the anti-PD-1 pembrolizumab (200 mg). The most frequent treatment-emergent adverse events were pyrexia, injection site reactions, and chills. Furthermore, early clinical efficacy was observed, including in immune checkpoint blockade-resistant/refractory patients.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Product Citations: 40
In Cell Reports Medicine on 18 February 2025 by Champiat, S., Garralda, E., et al.
In Stem Cell Research & Therapy on 20 May 2024 by Park, H. S., Lee, B. C., et al.
Mesenchymal stem cells (MSCs) play important roles in tissue homeostasis by providing a supportive microenvironmental niche for the hematopoietic system. Cigarette smoking induces systemic abnormalities, including an impeded recovery process after hematopoietic stem cell transplantation. However, the role of cigarette smoking-mediated alterations in MSC niche function have not been investigated.
In the present study, we investigated whether exposure to cigarette smoking extract (CSE) disrupts the hematopoietic niche function of MSCs, and pathways impacted. To investigate the effects on bone marrow (BM)-derived MSCs and support of hematopoietic stem and progenitor cells (HSPCs), mice were repeatedly infused with the CSE named 3R4F, and hematopoietic stem and progenitor cells (HSPCs) supporting function was determined. The impact of 3R4F on MSCs at cellular level were screened by bulk-RNA sequencing and subsequently validated through qRT-PCR. Specific inhibitors were treated to verify the ROS or NLRP3-specific effects, and the cells were then transplanted into the animal model or subjected to coculture with HSPCs.
Both direct ex vivo and systemic in vivo MSC exposure to 3R4F resulted in impaired engraftment in a humanized mouse model. Furthermore, transcriptomic profile analysis showed significantly upregulated signaling pathways related to reactive oxygen species (ROS), inflammation, and aging in 3R4F-treated MSCs. Notably, ingenuity pathway analysis revealed the activation of NLRP3 inflammasome signaling pathway in 3R4F-treated MSCs, and pretreatment with the NLRP3 inhibitor MCC950 rescued the HSPC-supporting ability of 3R4F-treated MSCs.
In conclusion, these findings indicate that exposure to CSE reduces HSPCs supportive function of MSCs by inducing robust ROS production and subsequent NLRP3 activation.
© 2024. The Author(s).
-
Stem Cells and Developmental Biology
A biomaterial platform for T cell-specific gene delivery.
In Acta Biomaterialia on 15 March 2024 by Pandit, S., Smith, B. E., et al.
Efficient T cell engineering is central to the success of CAR T cell therapy but involves multiple time-consuming manipulations, including T cell isolation, activation, and transduction. These steps add complexity and delay CAR T cell manufacturing, which takes a mean time of 4 weeks. To streamline T cell engineering, we strategically combine two critical engineering solutions - T cell-specific lentiviral vectors and macroporous scaffolds - that enable T cell activation and transduction in a simple, single step. The T cell-specific lentiviral vectors (referred to as STAT virus) target T cells through the display of an anti-CD3 antibody and the CD80 extracellular domain on their surface and provide robust T cell activation. Biocompatible macroporous scaffolds (referred to as Drydux) mediate robust transduction by providing effective interaction between naïve T cells and viral vectors. We show that when unstimulated peripheral blood mononuclear cells (PBMCs) are seeded together with STAT lentivirus on Drydux scaffolds, T cells are activated, selectively transduced, and reprogrammed in a single step. Further, we show that the Drydux platform seeded with PBMCs and STAT lentivirus generates tumor-specific functional CAR T cells. This potent combination of engineered lentivirus and biomaterial scaffold holds promise for an effective, simple, and safe avenue for in vitro and in vivo T cell engineering. STATEMENT OF SIGNIFICANCE: Manufacturing T cell therapies involves lengthy and labor-intensive steps, including T cell selection, activation, and transduction. These steps add complexity to current CAR T cell manufacturing protocols and limit widespread patient access to this revolutionary therapy. In this work, we demonstrate the combination of engineered virus and biomaterial platform that, together, enables selective T cell activation and transduction in a single step, eliminating multistep T cell engineering protocols and significantly simplifying the manufacturing process.
Copyright © 2024 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
-
FC/FACS
-
Biochemistry and Molecular biology
-
Immunology and Microbiology
Preprint on Research Square on 19 December 2023 by Weiner, L., FitzGerald, A., et al.
Natural killer (NK) cells play a critical role in physiologic and pathologic conditions such as pregnancy, infection, autoimmune disease and cancer. In cancer, numerous strategies have been designed to exploit the cytolytic properties of NK cells, with variable success. A major hurdle to NK-cell focused therapies is NK cell recruitment and infiltration into tumors. While the chemotaxis pathways regulating NK recruitment to different tissues are well delineated, the mechanisms human NK cells employ to physically migrate are ill-defined. We show for the first time that human NK cells express fibroblast activation protein (FAP), a cell surface protease previously thought to be primarily expressed by activated fibroblasts. FAP degrades the extracellular matrix to facilitate cell migration and tissue remodeling. We used novel in vivo zebrafish and in vitro 3D culture models to demonstrate that FAP knock out and pharmacologic inhibition restrict NK cell migration, extravasation, and invasion through tissue matrix. Notably, forced overexpression of FAP promotes NK cell invasion through matrix in both transwell and tumor spheroid assays, ultimately increasing tumor cell lysis. Additionally, FAP overexpression enhances NK cells invasion into a human tumor in immunodeficient mice. These findings demonstrate the necessity of FAP in NK cell migration and present a new approach to modulate NK cell trafficking and enhance cell-based therapy in solid tumors.
-
Cancer Research
In PeerJ on 14 May 2023 by Wang, Y., Sun, Z., et al.
The application of PD-1 monoclonal antibody (mAb) helps to treat non-small cell lung cancer, but acquired resistance has emerged in clinical practice. We tested the hypothesis that acquired resistance of anti-PD-1 immunotherapy is linked to death and exhaustion of activated T and NK cell.
The co-culture system of HCC827 cells and peripheral mononuclear cells (PBMCs) was established to evaluate the effect of PD-1 mAb on the death rate and exhaustion of T and NK cell. The predisposing role of CD69 for death and exhaustion was validated by using PHA-activated PBMCs of CD69low NSCLC patients. The 10-colour/three laser flow cytometer was used to test related markers for cell activation, death and exhaustion.
We found that PD-1 mAb increase the death and exhaustion of T cells and NK cells in a dose-dependent way when PBMCs from NSCLC patients whose the percentages of CD69+ cells in peripheral blood T cells were greater than 5% (CD69high NSCLC patients). By analyzing PBMCs from healthy volunteers and CD69low NSCLC patients, we found that T cells and NK cells can be induced to die by PD-1 mAb after PHA activation, and had a tendency to raise the rate of cell exhaustion.
Our findings imply that increased death and exhaustion of CD69high T cells and NK cells are associated with ineffective anti-PD-1 immunotherapy in lung cancer. The CD69 expression of T cells and NK cells may be developed as a potential predictor for acquired resistance of anti-PD-1 immunotherapy. These data may provide ideas to guide individualized medication of PD-1 mAb in NSCLC patients.
© 2023 Wang et al.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
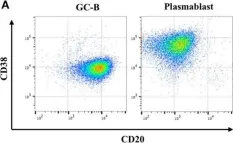
In Front Immunol on 14 August 2018 by Pak, H. K., Nam, B., et al.
Fig.1.A

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 1