The endothelium is the gatekeeper of vessel health, and its dysfunction is pivotal in driving atherogenesis. Here, we present a protocol to replicate endothelial-macrophage crosstalk during atherogenesis, called the "atherogenesis-on-chip" model, based on the Emulate dual-channel perfusion system. We describe a model for studying endothelial-macrophage interactions during atherogenesis in human aortic endothelial cells and human macrophages using qPCR and secretome analysis, fluorescence microscopy, and flow cytometry. This protocol could be adapted toward more complex plaque microenvironment or other disease settings.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Product Citations: 107
In STAR Protocols on 21 March 2025 by Hanford, K. M. L., Dzobo, K. E., et al.
-
Immunology and Microbiology
In European Journal of Immunology on 1 March 2025 by van Smoorenburg, M. Y., Remmerswaal, E. B. M., et al.
Young females are at high risk of acquiring HIV-1 infections and an imbalance in the vaginal microbiome enhances susceptibility to HIV-1 infection. More insights into the underlying mechanisms could open up new strategies to prevent HIV-1 acquisition and dissemination. Here, we investigated the effect of anaerobic bacteria associated with bacterial vaginosis (BV) on HIV-1 transmission by two distinct dendritic cell (DC) subsets, that is, inflammatory monocyte-derived DCs (moDCs) and primary CD1c+ DCs. Notably, in contrast to other BV-associated microbiota, Prevotella timonensis enhanced uptake of HIV-1 by both moDCs and CD1c+ DCs and the increased uptake was independent of cellular HIV-1 (co-)receptors. Imaging flow cytometry analyses showed that HIV-1 did not co-localise with P. timonensis but was internalized into tetraspanin-positive compartments known to be involved in HIV-1 transmission. P. timonensis bacteria enhanced HIV-1 transmission by CD1c+ DCs, but not by moDCs, and the enhanced transmission was independent of viral infection. Our study strongly suggests that mucosal DC subsets have distinct functions in BV-associated HIV-1 susceptibility, and underscores the importance of early diagnosis and targeted treatment of vaginal dysbiosis to reduce the risk of HIV-1 acquisition.
© 2025 The Author(s). European Journal of Immunology published by Wiley‐VCH GmbH.
-
Immunology and Microbiology
Guidelines for preparation and flow cytometry analysis of human nonlymphoid tissue DC.
In European Journal of Immunology on 1 January 2025 by Dudziak, D., Heger, L., et al.
This article is part of the Dendritic Cell Guidelines article series, which provides a collection of state-of-the-art protocols for the preparation, phenotype analysis by flow cytometry, generation, fluorescence microscopy, and functional characterization of mouse and human dendritic cells (DC) from lymphoid organs, and various nonlymphoid tissues. Within this article, detailed protocols are presented that allow for the generation of single-cell suspensions from human nonlymphoid tissues including lung, skin, gingiva, intestine as well as from tumors and tumor-draining lymph nodes with a subsequent analysis of dendritic cells by flow cytometry. Further, prepared single-cell suspensions can be subjected to other applications including cellular enrichment procedures, RNA sequencing, functional assays, etc. While all protocols were written by experienced scientists who routinely use them in their work, this article was also peer-reviewed by leading experts and approved by all co-authors, making it an essential resource for basic and clinical DC immunologists.
© 2024 The Author(s). European Journal of Immunology published by Wiley‐VCH GmbH.
-
Immunology and Microbiology
Skin Sensitization Potential of Sensitizers in the Presence of Metal Oxide Nanoparticles In Vitro.
In Nanomaterials (Basel, Switzerland) on 12 November 2024 by Meindl, C., Öhlinger, K., et al.
Silica (SiO2), titanium dioxide (TiO2), and zinc oxide (ZnO) nanoparticles (NPs) are widely used in dermal products. Their skin sensitization potential, especially their effects in combination with known sensitizers, is poorly studied in vitro and their sensitization inconsistently reported in animal studies. In this study, cellular assays were used to identify different steps of sensitization, the activation of keratinocytes and dendritic cells, when cells were exposed to these NPs in the absence and presence of sensitizers. Cellular systems included HaCaT keratinocytes and U937 (U-SENS™) alone, as well as different co-culture systems of THP-1 cells with HaCaT cells (COCAT) and with primary keratinocytes. The effect of NPs differed between co-cultures and U-SENS™, whereas co-cultures with either primary keratinocytes or HaCaT cells responded similarly. Pre-exposure to ZnO NPs increased the U-SENS™ assay response to 2,4-dinitrochlorobenzene six-fold. The COCAT increase was maximally four-fold for the combination of SiO2 and trans cinnamaldehyde. When the THP-1 cells were separated from the keratinocytes by a membrane, the response of the co-culture system was more similar to U-SENS™. The direct contact with keratinocytes decreased the modulating effect of TiO2 and ZnO NPs but suggested an increase in response to sensitizers following dermal contact with SiO2 NPs.
AMPK activation induces RALDH+ tolerogenic dendritic cells by rewiring glucose and lipid metabolism.
In The Journal of Cell Biology on 7 October 2024 by Brombacher, E. C., Patente, T. A., et al.
Dendritic cell (DC) activation and function are underpinned by profound changes in cellular metabolism. Several studies indicate that the ability of DCs to promote tolerance is dependent on catabolic metabolism. Yet the contribution of AMP-activated kinase (AMPK), a central energy sensor promoting catabolism, to DC tolerogenicity remains unknown. Here, we show that AMPK activation renders human monocyte-derived DCs tolerogenic as evidenced by an enhanced ability to drive differentiation of regulatory T cells, a process dependent on increased RALDH activity. This is accompanied by several metabolic changes, including increased breakdown of glycerophospholipids, enhanced mitochondrial fission-dependent fatty acid oxidation, and upregulated glucose catabolism. This metabolic rewiring is functionally important as we found interference with these metabolic processes to reduce to various degrees AMPK-induced RALDH activity as well as the tolerogenic capacity of moDCs. Altogether, our findings reveal a key role for AMPK signaling in shaping DC tolerogenicity and suggest AMPK as a target to direct DC-driven tolerogenic responses in therapeutic settings.
© 2024 Brombacher et al.
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
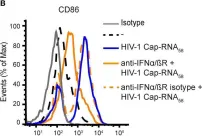
In Front Immunol on 11 February 2020 by Stunnenberg, M., Sprokholt, J. K., et al.
Fig.2.B

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 2
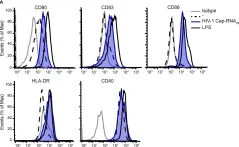
In Front Immunol on 11 February 2020 by Stunnenberg, M., Sprokholt, J. K., et al.
Fig.2.A

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 2