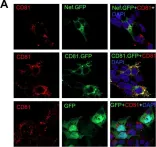
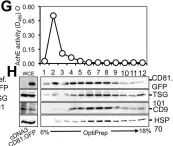
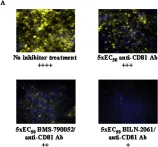
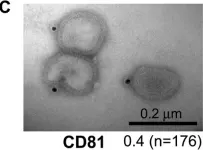
Tumor-derived extracellular vesicles (EVs) have attracted significant attention, yet the molecular mechanisms that govern their specific binding to recipient cells remain elusive. Our in vitro study utilizing single-particle tracking demonstrated that integrin heterodimers comprising α6β4 and α6β1 and ganglioside, GM1, are responsible for the binding of small EV (sEV) subtypes to laminin. EVs derived from four distinct tumor cell lines, regardless of size, exhibited high binding affinities for laminin but not for fibronectin, although fibronectin receptors are abundant in EVs and have functional roles in EV-secreting cells. Our findings revealed that integrins in EVs bind to laminin via the conventional molecular interface, facilitated by CD151 rather than by inside-out signaling of talin-1 and kindlin-2. Super-resolution movie observation revealed that sEV integrins bind only to laminin on living recipient cells. Furthermore, sEVs bound to HUVEC and induced cell branching morphogenesis in a laminin-dependent manner. Thus, we demonstrated that EVs predominantly bind to laminin on recipient cells, which is indispensable for cell responses.
© 2025 Isogai et al.
Product Citations: 180
Extracellular vesicles adhere to cells primarily by interactions of integrins and GM1 with laminin.
In The Journal of Cell Biology on 2 June 2025 by Isogai, T., Hirosawa, K. M., et al.
-
Cell Biology
In JHEP Reports (Online) on 1 May 2025 by Sheldon, J. A., Winkler, M., et al.
Hepatitis C virus (HCV) has a narrow species tropism and cannot infect mice. To understand HCV species tropism and to develop better animal models, we adapted HCV to infect mouse cells deficient in innate immunity and with minimal human HCV host factors.
HCV was adapted via passaging an HCV infectious virus clone several times in human hepatoma cells, mouse liver cells, and eventually primary mouse hepatocytes deficient in innate immunity and ectopically expressing human occludin and human CD81. Using RNAseq the sequence of the adapted virus was analyzed, and several clones were generated to study replication and infection kinetics as well as neutralization assays in several human/mouse cell lines and primary hepatocytes from human, mouse, and macaques.
Accumulation of 35 non-synonymous and 66 synonymous mutations correlated with >1,000-fold enhanced production of infectious progeny from primary mouse hepatocytes. These mutations did not confer drug resistance or evasion from innate immunity. They did not enhance fitness in human or macaque hepatocytes. We show that non-synonymous mutations are necessary and sufficient for adaptation, and that changes to the glycoproteins are not essential. Mutations outside of viral envelope proteins enhanced specific infectivity and facilitated viral spread in murine cells.
This study reveals key viral factors governing HCV species tropism. The mouse-adapted HCV opens up possibilities for the development of animal models to analyze HCV pathogenesis, immune control, and vaccine development.
This work demonstrates the feasibility in principle of HCV adaptation to replication in and infection of non-human cells. This is made possible by a manageable number of non-synonymous mutations and opens up new ways to elucidate the principles of HCV species tropism and to develop important animal models for HCV research in the long term.
© 2025 The Authors.
-
Immunology and Microbiology
In Scientific Reports on 1 March 2025 by Sulaiman, E., Yellon, D. M., et al.
Small extracellular vesicles (sEV) are nanosized vesicles that facilitate intracellular communication. A significant research obstacle is the isolation of sEV devoid of non-sEV contaminants. Immunoaffinity capture with sEV-specific antibodies is an attractive approach to purifying sEV, but it risks disrupting the vesicles during antibody dissociation. Furthermore, immunoaffinity capture may require the modification of EV-specific proteins for the incorporation of tags on the EV surface, with unknown implications on EV production and function. The aim of this study was to investigate whether a previously reported CD63 truncation is efficient for the incorporation of small tags on the extravesicular surface. We therefore conjugated ALFA-tag to N-terminal-truncated CD63, and included nanoluciferase at the C-terminus, for luminescent tracing of the sEV. Full-length CD63-nanoluciferase was used as a control. Plasmid constructs expressing these proteins were transfected into HEK293 cells. In contrast to a previous report, the N-terminal truncation of CD63 impaired its membrane localisation and reduced the yield of EVs. Further investigation revealed that some of the tagged CD63 was co-localized with aggresomes and was preferentially secreted from the cells as soluble protein rather than being associated with sEV. These results demonstrate that CD63 truncation can impair its function and EV yield, potentially generating misleading results.
© 2025. The Author(s).
-
Homo sapiens (Human)
In Cellular and Molecular Life Sciences : CMLS on 13 February 2025 by Picard, F., Nonaka, T., et al.
Proteinopathies, such as amyotrophic lateral sclerosis (ALS), are marked by the accumulation of misfolded proteins that disrupt cellular processes. Eukaryotic cells have developed protein quality control systems to eliminate these aberrant proteins, but these systems often fail to differentiate between normal and misfolded proteins. In ALS, pathological inclusions primarily composed of misfolded TDP-43 are a hallmark of the disease. Recently, a novel unconventional secretion process called misfolding-associated protein secretion (MAPS) has been discovered to selectively export misfolded proteins. USP19, an Endoplasmic Reticulum-associated ubiquitin peptidase, plays a crucial role in this process. In this study, we investigated the impact of ER-anchored USP19 on the secretion of misfolded TDP-43. Here we found that USP19 overexpression significantly promotes the secretion of soluble and aggregated misfolded TDP-43, requiring both ER anchoring and ubiquitin peptidase activity. Characterization of the cellular and molecular mechanisms involved in this process highlighted the importance of early autophagosomal and late endosomal/amphisomal compartments, while lysosomes did not play a key role. By using dominant-negative mutants and small interfering RNAs, we identified that USP19-mediated secretion of misfolded TDP-43 is modulated by key factors involved in cellular trafficking and secretion pathways, such as ATG7, the ESCRT-O HGS/HRS, the Rab GTPases RAB11A, RAB8A, and RAB27A, and the v-SNARE VAMP7. We also confirmed the crucial role of the DNAJC5/CSPα cochaperone. Overall, this study provides new insights into how cells manage the secretion of misfolded TDP-43 proteins and potentially opens new avenues for therapeutic interventions in ALS and related disorders.
© 2025. The Author(s).
-
Biochemistry and Molecular biology
In Science Advances on 3 January 2025 by Choi, Y., Park, J. H., et al.
The early detection of neurodegenerative diseases necessitates the identification of specific brain-derived biomolecules in peripheral blood. In this context, our investigation delineates the role of amyloid precursor-like protein 1 (APLP1)-a protein predominantly localized in oligodendrocytes and neurons-as a previously unidentified biomarker in extracellular vesicles (EVs). Through rigorous analysis, APLP1+ EVs from human sera were unequivocally determined to be of cerebral origin. This assertion was corroborated by distinctive small RNA expression patterns of APLP1+ EVs. The miRNAs' putative targets within these EVs manifested pronounced expression in the brain, fortifying their neurospecific provenance. We subjected our findings to stringent validation using Thy-1 GFP M line mice, transgenic models wherein GFP expression is confined to hippocampal neurons. An amalgamation of these results with an exhaustive data analysis accentuates the potential of APLP1+ EVs as cerebrally originated biomarkers. Synthesizing our findings, APLP1+ EVs are postulated not merely as diagnostic markers but as seminal entities shaping the future trajectory of neurodegenerative disease diagnostics.
-
Cardiovascular biology
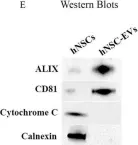
In Front Aging Neurosci on 10 July 2023 by Attaluri, S., Jaimes Gonzalez, J., et al.
Fig.1.E

-
WB
-
Collected and cropped from Front Aging Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 17
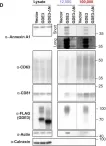
In Sci Adv on 23 June 2023 by Levy-Myers, R., Daudelin, D., et al.
Fig.1.D

-
WB
-
Collected and cropped from Sci Adv by CiteAb, provided under a CC-BY license
Image 1 of 17
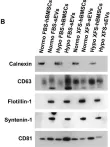
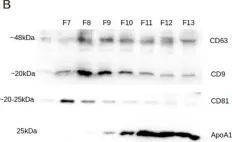
In Theranostics on 15 April 2023 by Palamà, M. E. F., Coco, S., et al.
Fig.2.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Theranostics by CiteAb, provided under a CC-BY license
Image 1 of 17
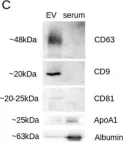
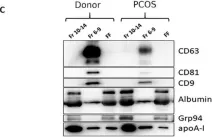
In Int J Mol Sci on 4 April 2022 by Lattekivi, F., Guljavina, I., et al.
Fig.1.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 17
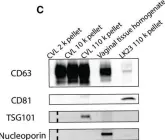
In Int J Mol Sci on 4 April 2022 by Lattekivi, F., Guljavina, I., et al.
Fig.1.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 17
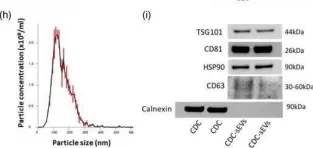
In J Extracell Vesicles on 1 January 2022 by Ciullo, A., Li, C., et al.
Fig.1.H

-
WB
-
Collected and cropped from J Extracell Vesicles by CiteAb, provided under a CC-BY license
Image 1 of 17
In Int J Mol Sci on 15 December 2020 by Rooda, I., Hasan, M. M., et al.
Fig.2.C

-
WB
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 17
In FEBS Open Bio on 1 October 2020 by Zhao, Z., Muth, D. C., et al.
Fig.1.C

-
WB
-
Collected and cropped from FEBS Open Bio by CiteAb, provided under a CC-BY license
Image 1 of 17
In Cells on 27 November 2019 by Barilani, M., Peli, V., et al.
Fig.2.H

-
WB
-
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 17
In Cells on 27 November 2019 by Barilani, M., Peli, V., et al.
Fig.2.G

-
WB
-
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 17
In Front Immunol on 1 May 2018 by Cai, C., Koch, B., et al.
Fig.4.A

-
WB
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 17
In PLoS One on 29 April 2015 by Luo, X., Fan, Y., et al.
Fig.10.B

-
ICC-IF
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 17
In PLoS One on 29 April 2015 by Luo, X., Fan, Y., et al.
Fig.10.A

-
ICC-IF
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 17
In PLoS One on 29 April 2015 by Luo, X., Fan, Y., et al.
Fig.8.G

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 17
In PLoS One on 12 June 2013 by Bush, C. O., Greenstein, A. E., et al.
Fig.1.A

-
ICC-IF
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 17
In Retrovirology on 14 July 2009 by Krementsov, D. N., Weng, J., et al.
Fig.1.A

-
WB
-
Collected and cropped from Retrovirology by CiteAb, provided under a CC-BY license
Image 1 of 17
In PLoS Pathog on 6 June 2008 by Shaw, M. L., Stone, K. L., et al.
Fig.5.C

-
ICC
-
Influenza A virus (A/Taiwan/01/1986(H1N1))
Collected and cropped from PLoS Pathog by CiteAb, provided under a CC-BY license
Image 1 of 17