The ECM (extracellular matrix) provides the microenvironmental niche sensed by resident vascular smooth muscle cells (VSMCs). Aging and disease are associated with dramatic changes in ECM composition and properties; however, their impact on VSMC phenotype remains poorly studied.
Here, we describe a novel in vitro model system that utilizes endogenous ECM to study how modifications associated with age and metabolic disease impact VSMC phenotype. ECM was synthesized using primary human VSMCs and modified during culture or after decellularization. Integrity, stiffness, and composition of the ECM was measured using superresolution microscopy, atomic force microscopy, and proteomics, respectively. VSMCs reseeded onto the modified ECM were analyzed for viability and osteogenic differentiation.
ECMs produced in response to mineral stress showed extracellular vesicle-mediated hydroxyapatite deposition and sequential changes in collagen composition and ECM properties. VSMCs seeded onto the calcified ECM exhibited increased extracellular vesicle release and Runx2 (Runt-related transcription factor 2)-mediated osteogenic gene expression due to the uptake of hydroxyapatite, which led to increased reactive oxygen species and the induction of DNA damage signaling. VSMCs seeded onto the nonmineralized, senescent ECM also exhibited increased Runx2-mediated osteogenic gene expression and accelerated calcification. In contrast, glycated ECM specifically induced increased ALP (alkaline phosphatase) activity, and this was dependent on RAGE (receptor for advanced glycation end products) signaling with both ALP and RAGE receptor inhibition attenuating calcification.
ECM modifications associated with aging and metabolic disease can directly induce osteogenic differentiation of VSMCs via distinct mechanisms and without the need for additional stimuli. This highlights the importance of the ECM microenvironment as a key driver of phenotypic modulation acting to accelerate age-associated vascular pathologies and provides a novel model system to study the mechanisms of calcification.
Product Citations: 27
In Arteriosclerosis, Thrombosis, and Vascular Biology on 1 March 2025 by Whitehead, M., Faleeva, M., et al.
-
Biochemistry and Molecular biology
-
Cardiovascular biology
-
Cell Biology
Exosome Loaded in Microneedle Patch Ameliorates Renal Ischemia-Reperfusion Injury in a Mouse Model.
In Stem Cells International on 23 January 2025 by Taghavi, S., Keshtkar, S., et al.
Introduction: Renal dysfunction due to ischemia-reperfusion injury (IRI) is a common problem after kidney transplantation. In recent years, studies on animal models have shown that exosomes derived from mesenchymal stem cells (MSC-Exo) play an important role in treating acute kidney injury (AKI) and promoting tissue repair. The microneedle patch provides a noninvasive and targeted delivery system for exosomes. The purpose of this innovative approach is to combine MSC-Exo with microneedle patches. Method: Exosomes were isolated from MSCs, characterized, and placed in the prepared microneedle patch. Then this construct was applied to the IRI mice model. After 7 days, the gene expression of miR-34a and its targets B-cell lymphoma-2 (BCL-2) and BCL-2-associated X (BAX), along with reactive oxygen species (ROS) and lipid peroxidation (LPO) production, was investigated. Additionally, renoprotection was evaluated for measuring blood urea nitrogen (BUN) and creatinine (Cr) and histopathology detection. Results: After using microneedle patches containing exosomes, the reduction of miR-34a and BAX and enhancement of BCL-2 were observed. Moreover, treatment by this construct decreased the production of ROS, LPO, BUN, and Cr and improved tissue damage. Conclusion: The use of a microneedle patch containing exosomes is a noninvasive method that enables the release of exosomes in a slow manner. In comparison to exosome injection alone, microneedle patch-exosome treatment offers a longer and more targeted effect that improves renal IRI dysfunction and reduces tissue damage, potentially facilitating the clinical application of exosomes and improving graft survival.
Copyright © 2025 Samin Taghavi et al. Stem Cells International published by John Wiley & Sons Ltd.
-
Stem Cells and Developmental Biology
In BMC Cancer on 21 November 2024 by Mo, J. L., Li, X., et al.
Exosome small RNAs are believed to be involved in the pathogenesis of cancer, but their role in breast cancer is still unclear. This study utilized machine learning models to screen for key exosome small RNAs and analyzed and validated them.
Peripheral blood samples from breast cancer screening positive and negative people were used for small RNA sequencing of plasma exosomes. The differences in the expression of small RNAs between the two groups were compared. We used machine learning algorithms to analyze small RNAs with significant differences between the two groups, fit the model through training sets, and optimize the model through testing sets. We recruited new research subjects as validation samples and used PCR-based quantitative detection to validate the key small RNAs screened by the machine learning model. Finally, target gene prediction and functional enrichment analysis were performed on these key RNAs.
The machine learning model incorporates six small RNAs: piR-36,340, piR-33,161, miR-484, miR-548ah-5p, miR-4282, and miR-6853-3p. The area under the ROC curve (AUC) of the machine learning model in the training set was 0.985 (95% CI = 0.948-1), while the AUC in the test set was 0.972 (95% CI = 0.882-0.995). RT-qPCR was used to detect the expression levels of these key small RNAs in the validation samples, and the results revealed that their expression levels were significantly different between the two groups (P < 0.05). Through target gene prediction and functional enrichment analysis, it was found that the functions of the target genes were enriched mainly in the chemokine signaling pathway.
The combination of six plasma exosome small RNAs has good prognostic value for women with positive breast cancer by imaging screening. The chemokine signaling pathway may be involved in the early stage of breast cancer. It is worth further exploring whether small RNAs mediate chemokine signaling pathways in the pathogenesis of breast cancer through the delivery of exosomes.
© 2024. The Author(s).
-
FC/FACS
-
Homo sapiens (Human)
-
Cancer Research
-
Genetics
In Molecular Carcinogenesis on 1 October 2024 by Zhou, X., Tong, Y., et al.
Cancer-associated fibroblasts (CAFs) are abundant and heterogeneous stromal cells in the tumor microenvironment, which play important roles in regulating tumor progression and therapy resistance by transferring exosomes to cancer cells. However, how CAFs modulate esophageal squamous cell carcinoma (ESCC) progression and radioresistance remains incompletely understood. The expression of fibroblast activation protein (FAP) in CAFs was evaluated by immunohistochemistry in 174 ESCC patients who underwent surgery and 78 pretreatment biopsy specimens of ESCC patients who underwent definitive chemoradiotherapy. We sorted CAFs according to FAP expression, and the conditioned medium (CM) was collected to culture ESCC cells. The expression levels of several lncRNAs that were considered to regulate ESCC progression and/or radioresistance were measured in exosomes derived from FAP+ CAFs and FAP- CAFs. Subsequently, cell counting kit-8, 5-ethynyl-2'-deoxyuridine, transwell, colony formation, and xenograft assays were performed to investigate the functional differences between FAP+ CAFs and FAP- CAFs. Finally, a series of in vitro and in vivo assays were used to evaluate the effect of AFAP1-AS1 on radiosensitivity of ESCC cells. FAP expression in stromal CAFs was positively correlated with nerve invasion, vascular invasion, depth of invasion, lymph node metastasis, lack of clinical complete response and poor survival. Culture of ESCC cells with CM/FAP+ CAFs significantly increased cancer proliferation, migration, invasion and radioresistance, compared with culture with CM/FAP- CAFs. Importantly, FAP+ CAFs exert their roles by directly transferring the functional lncRNA AFAP1-AS1 to ESCC cells via exosomes. Functional studies showed that AFAP1-AS1 promoted radioresistance by enhancing DNA damage repair in ESCC cells. Clinically, high levels of plasma AFAP1-AS1 correlated with poor responses to dCRT in ESCC patients. Our findings demonstrated that FAP+ CAFs promoted radioresistance in ESCC cells through transferring exosomal lncRNA AFAP1-AS1; and may be a potential therapeutic target for ESCC treatment.
© 2024 Wiley Periodicals LLC.
-
Cancer Research
In Cells on 24 August 2024 by Shuler, K. T., Llamas-Rodríguez, J., et al.
Extracellular vesicles (EVs) are implicated in a multitude of physiological and pathophysiological processes in the nervous system; however, their biogenesis and cargoes are not well defined. Glycerophosphodiester Phosphodiesterase 2 (GDE2 or GDPD5) is a six-transmembrane protein that cleaves the Glycosylphosphatidylinositol (GPI)-anchor that tethers some proteins to the membrane and has important roles in neurodevelopment and disease-relevant pathways of neuronal survival. We show here that GDE2 regulates the number of small EVs (sEVs) released from the cell surface of neurons via its GPI-anchor cleavage activity and contributes to the loading of protein cargo through enzymatic and non-enzymatic mechanisms. Proteomic profiling reveals that GDE2 releases at least two distinct EV populations, one containing GDE2 itself and the other harboring the putative ectosomal markers CD9 and BSG. sEVs released by GDE2 are enriched in cytoskeletal and actin-remodeling proteins, suggesting a potential mechanism for GDE2-dependent EV release. Further, sEV populations released by GDE2 are enriched in proteins responsible for modulating synaptic activity and proteins that are critical for cellular redox homeostasis. These studies identify GDE2 as a novel regulator of molecularly distinct sEV populations from neurons with potential roles in the synaptic and redox pathways required for neuronal function and survival.
-
Cell Biology
-
Neuroscience
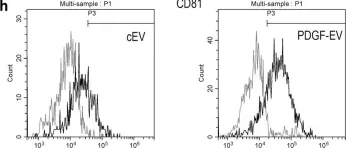
In Sci Rep on 4 December 2018 by Lopatina, T., Favaro, E., et al.
Fig.1.H

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 1