Since T cells are key mediators in the adaptive immune system, evaluating antigen-specific T cell immune responses is pivotal to understanding immune function. Commonly used methods for measuring T cell responses include Activation-Induced Marker (AIM) assays and Intracellular Cytokine Staining (ICS). However, combining these approaches has rarely been reported. This study describes a combined AIM + ICS assay and the effect of collecting the supernatant. Peripheral blood mononuclear cells (PBMCs) from seven healthy donors were stimulated with DMSO (negative control), Epstein-Barr virus (EBV) peptide pools, and PHA (positive control). The AIM markers OX40 + CD137+ were used for CD4+ T cells and CD69 + CD137+ and CD107a + CD137+ for CD8+ T cells. Cytokine-secreting cells were identified as CD40L+ cytokine+ for CD4+ and CD69+ or CD107 + cytokine+ for CD8+ T cells. Half of the supernatant was collected before adding the BFA/Monensin/CD137 antibody solution to assess the impact on T cell responses. The CD107a + CD137+ AIM markers combination had a lower background than CD69 + CD137+, making CD107a+ a more sensitive marker for CD8+ AIM markers. Collecting half of the supernatant did not significantly affect the immune responses. Our AIM + ICS combined protocol enables the simultaneous assessment of activation and cytokine release reducing the sample volume for testing T cell responses. We also show that collecting half of the supernatant does not significantly interfere with immune responses detection.
© The Author(s) 2024. Published by Oxford University Press.
Product Citations: 49
In Oxford Open Immunology on 23 December 2024 by Lee, Y. J., Tarke, A., et al.
-
Immunology and Microbiology
In IScience on 18 October 2024 by Kabakibo, T. S., Arnold, E., et al.
T cell immune dysfunction is a prominent feature of chronic HIV infection. To evaluate non-specific dysfunction, a method involving both generic activation and T cell receptor (TCR) stimulation is necessary. We created a tunable artificial antigen-presenting cell (aAPC) system. This system consists of lipid bilayers on cytometry-compatible silica microbeads (5 μm). When only anti-CD3 is incorporated, T cell activation is limited. Introducing anti-CD28 agonists significantly elevates the cytokine expression and upregulation of activation-induced markers. CD28 co-stimulation modulates the response profile, preferentially promoting IL-2 expression relative to other cytokines. aAPCs-stimulated CD4+ and CD8+ T cells from untreated HIV-infected individuals exhibit altered effector functions and diminished CD28 dependence. These functions are skewed toward TNFα, IFNγ and CD107a, with reduced IL-2. Antiretroviral therapy partially normalizes this distorted profile in CD4+ T cells, but not in CD8+ T cells. Our findings show T cell intrinsic biases that may contribute to persistent systemic T cell dysfunction associated with HIV pathogenesis.
© 2024 The Author(s).
-
Immunology and Microbiology
In Journal of Immunological Methods on 1 August 2024 by Pedersen, K., Laursen, N. S., et al.
When the membrane protein CD40 ligand (CD40L) on activated T cells binds the receptor CD40 on B-cells, it provides a co-stimulatory signal for B cell activation. Dysregulation of the CD40L:CD40 axis is associated with inflammatory and autoimmune diseases. The presence of soluble CD40L (sCD40L) in plasma is implicated in several diseases, from cardiovascular and autoimmune diseases to different types of cancer, and sCD40L has been suggested as a valuable marker of disease. If sCD40L is to be used as a biomarker, being able to precisely measure and quantify the levels of sCD40L in human blood samples is of utmost importance. We demonstrate the development of a sandwich-type time-resolved immunofluorometric assay for quantification of sCD40L in plasma or serum samples. For this, we generate 29 monoclonal anti-CD40L antibodies, and from these, we select the optimal combination of capture antibody and detection antibody. A number of variables were tested: the influence of the type of sample (comparing 3 different blood collection tubes for serum sampling and 4 different types of tubes for plasma sampling), the influence of freeze-thaw cycles, the influence of sampling time during night and day, and the influence of centrifugation of the samples. We found a very similar level of sCD40L in paired EDTA plasma and serum samples. Out of 100 healthy blood donor samples 61 had a level of sCD40L below the detection level of the assay, whereas the remaining 39 samples had ranging levels of sCD40L from 1.14 to 33.14 ng/mL. In summary, we present a time-resolved immunofluorometric assay based on paired monoclonal antibodies, ensuring high specificity, sensitivity, and homogeneity. The Eu3+-based assay additionally provides consistent assay readouts due to the extended decay time not seen in standard enzyme-linked immunosorbent assays. The assay paves the way for specific and consistent quantification of sCD40L in human plasma and serum samples.
Copyright © 2024. Published by Elsevier B.V.
-
Immunology and Microbiology
In Nature Immunology on 1 August 2024 by Sun, M., Phan, J. M., et al.
A subset of individuals exposed to Mycobacterium tuberculosis (Mtb) that we refer to as 'resisters' (RSTR) show evidence of IFN-γ- T cell responses to Mtb-specific antigens despite serially negative results on clinical testing. Here we found that Mtb-specific T cells in RSTR were clonally expanded, confirming the priming of adaptive immune responses following Mtb exposure. RSTR CD4+ T cells showed enrichment of TH17 and regulatory T cell-like functional programs compared to Mtb-specific T cells from individuals with latent Mtb infection. Using public datasets, we showed that these TH17 cell-like functional programs were associated with lack of progression to active tuberculosis among South African adolescents with latent Mtb infection and with bacterial control in nonhuman primates. Our findings suggested that RSTR may successfully control Mtb following exposure and immune priming and established a set of T cell biomarkers to facilitate further study of this clinical phenotype.
© 2024. The Author(s).
-
Immunology and Microbiology
In The Journal of Clinical Investigation on 1 February 2024 by Kuehn, H. S., Sakovich, I. S., et al.
AIOLOS, also known as IKZF3, is a transcription factor that is highly expressed in the lymphoid lineage and is critical for lymphocyte differentiation and development. Here, we report on 9 individuals from 3 unrelated families carrying AIOLOS variants Q402* or E82K, which led to AIOLOS haploinsufficiency through different mechanisms of action. Nonsense mutant Q402* displayed abnormal DNA binding, pericentromeric targeting, posttranscriptional modification, and transcriptome regulation. Structurally, the mutant lacked the AIOLOS zinc finger (ZF) 5-6 dimerization domain, but was still able to homodimerize with WT AIOLOS and negatively regulate DNA binding through ZF1, a previously unrecognized function for this domain. Missense mutant E82K showed overall normal AIOLOS functions; however, by affecting a redefined AIOLOS protein stability domain, it also led to haploinsufficiency. Patients with AIOLOS haploinsufficiency showed hypogammaglobulinemia, recurrent infections, autoimmunity, and allergy, but with incomplete clinical penetrance. Altogether, these data redefine the AIOLOS structure-function relationship and expand the spectrum of AIOLOS-associated diseases.
-
Immunology and Microbiology
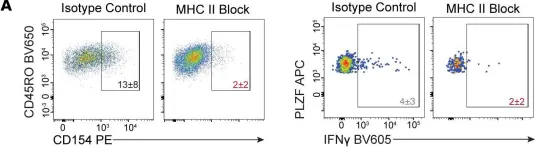
In JCI Insight on 22 November 2023 by Locher, V., Park, S., et al.
Fig.5.A

-
FC/FACS
-
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 5
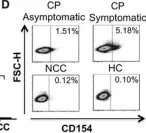
In Immun Inflamm Dis on 1 March 2018 by Ripoll, J. G., Giraldo, N. A., et al.
Fig.2.D

-
FC/FACS
-
Collected and cropped from Immun Inflamm Dis by CiteAb, provided under a CC-BY license
Image 1 of 5
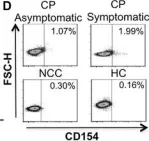
In Immun Inflamm Dis on 1 March 2018 by Ripoll, J. G., Giraldo, N. A., et al.
Fig.3.D

-
FC/FACS
-
Collected and cropped from Immun Inflamm Dis by CiteAb, provided under a CC-BY license
Image 1 of 5
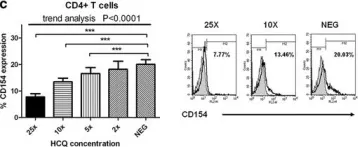
In Arthritis Res Ther on 9 August 2017 by Wu, S. F., Chang, C. B., et al.
Fig.2.C

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Arthritis Res Ther by CiteAb, provided under a CC-BY license
Image 1 of 5
In Arthritis Res Ther on 9 August 2017 by Wu, S. F., Chang, C. B., et al.
Fig.6.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Arthritis Res Ther by CiteAb, provided under a CC-BY license
Image 1 of 5