The SARS-CoV-2 index-virus nanoparticle protein vaccine (NVX-CoV2373) induces humoral and cell-mediated immune responses that protect against severe COVID-19, including from SARS-CoV-2 variants. Limited information exists on NVX-CoV2373-induced cell-mediated immune responses to ancestral SARS-CoV-2 and the Omicron variant following a homologous booster (third dose), and on T-cell responses following a booster dose compared to a single dose.
T-cell responses were investigated in participants from a randomised, placebo-controlled, phase 2A/2B trial of NVX-CoV2373 in South Africa, who had a blinded crossover at 6 months post-enrolment. Peripheral blood mononuclear cells were available for 34 participants, 7 days post-vaccination with one NVX-CoV2373 dose (n = 17) or a homologous booster (n = 17). T-cell responses to the full-length spike (FLS) glycoprotein of ancestral Wuhan-Hu-1 SARS-CoV-2 and mutated spike protein regions found in Omicron (BA.4/BA.5) were characterised by intracellular cytokine staining.
Here we show that FLS-specific T-cell responses are similar between single-dose and booster-dose recipients (CD4+: p = 0.871; CD8+: p = 0.491) and are predominantly monofunctional (IFN-γ or TNF-α). A third NVX-CoV2373 dose increases the FLS-specific polyfunctional cytokine production profile of CD4+ T cells compared with after a single dose (p = 0.045), whereas CD8+ T cells remain unaffected (p = 0.462). Only CD4+ T cells exhibit reduced reactivity to Omicron compared with ancestral SARS-CoV-2 in single-dose (p = 0.010) and in booster-dose recipients (p = 0.028).
NVX-CoV2373-induced T-cell responses to ancestral SARS-CoV-2 are comparable following vaccination with a single dose compared with a third dose administered 6 months after the second dose. Our findings suggest that an NVX-CoV2373 booster dose does not enhance T-cell immunity. Furthermore, NVX-CoV2373 vaccination induces greater T-cell response magnitudes to ancestral SARS-CoV-2, from which the vaccine is derived, compared with the Omicron variant.
© 2025. The Author(s).
Product Citations: 413
In Commun Med (Lond) on 10 June 2025 by McMahon, W. C., Kwatra, G., et al.
-
COVID-19
-
Immunology and Microbiology
Celluloepidemiology-A paradigm for quantifying infectious disease dynamics on a population level.
In Science Advances on 16 May 2025 by Ha, M., Postovskaya, A., et al.
To complement serology as a tool in public health interventions, we introduced the "celluloepidemiology" paradigm where we leveraged pathogen-specific T cell responses at a population level to advance our epidemiological understanding of infectious diseases, using SARS-CoV-2 as a model. Applying flow cytometry and machine learning on data from more than 500 individuals, we showed that the number of T cells with positive expression of functional markers not only could distinguish patients who recovered from COVID-19 from controls and pre-COVID donors but also identify previously unrecognized asymptomatic patients from mild, moderate, and severe recovered patients. The celluloepidemiology approach was uniquely capable to differentiate health care worker groups with different SARS-CoV-2 exposures from each other. T cell receptor (TCR) profiling strengthened our analysis by revealing that SARS-CoV-2-specific TCRs were more abundant in patients than in controls. We believe that adding data on T cell reactivity will complement serology and augment the value of infection morbidity modeling for populations.
T-bet+CD8+ T cells govern anti-PD-1 responses in microsatellite-stable gastric cancers.
In Nature Communications on 25 April 2025 by Tang, S., Che, X., et al.
More than 90% of advanced gastric cancers (GC) are microsatellite-stable (MSS). Compared to the high response rate of immune checkpoint inhibitors (ICI) in microsatellite-instability-high (MSI-H) GCs, only 10% of unstratified MSS GCs respond to ICIs. In this study, we apply semi-supervised learning to stratify potential ICI responders in MSS GCs, achieving high accuracy, quantified by an area under the curve of 0.924. Spatial analysis of the tumor microenvironment of ICI-sensitive GCs reveals a high level of T-bet+ CD8 + T cell infiltration in their tumor compartments. T-bet+ CD8 + T cells exhibit superior anti-tumor activity due to their increased ability to infiltrate tumors and secrete cytotoxic molecules. Adoptive transfer of T-bet+ CD8 + T cells boosts anti-tumor immunity and confers susceptibility to ICIs in immune-ignorant MSS GCs in a humanized mouse model. Spatial RNA sequencing suggests a positive-feedback loop between T-bet+ T cells and PD-L1+ tumor cells, which eventually drives T cell exhaustion and can therefore be leveraged for ICI therapy. In summary, our research provides insights into the underlying mechanism of anti-tumor immunity and deepens our understanding of varied ICI responses in MSS GCs.
© 2025. The Author(s).
-
Immunology and Microbiology
In STAR Protocols on 21 March 2025 by Rotta, G., Achille, V., et al.
Flow cytometry characterization of antigen-specific polyfunctional T cells is a valuable tool to study adaptive immunity. Here, we present a protocol for flow cytometry immunophenotyping of human antigen-specific T cells by activation-induced marker (AIM) and Th1 cytokine detection. We describe steps for preparing peripheral blood mononuclear cells (PBMCs) for stimulation followed by washing and staining PBMCs for flow cytometry. We then detail procedures for acquisition and analysis. This protocol has potential applications in the field of vaccine immunology and immuno-oncology. For complete details on the use and execution of this protocol, please refer to Altosole et al.1.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Immunology and Microbiology
In Frontiers in Immunology on 21 February 2025 by Clegg, J., Mnich, M. E., et al.
Staphylococcus aureus poses an enormous burden of morbidity and mortality worldwide. Making an efficacious vaccine has however proven extremely challenging. Due to colonizing interactions, pre-existing S. aureus-specific CD4+ T cells are often found in the human population and yet a detailed characterization of their phenotypes and how they might in turn impact vaccine efficacy are thus far unknown. Using an activation induced marker assay to sort for S. aureus-specific CD4+ T cells in an effector function-independent manner, single cell transcriptomic analysis was conducted. Remarkably, S. aureus-specific CD4+ T cells consisted not only of a broader spectrum of conventional T cells (Tcon) than previously described but also of regulatory T cells (Treg). As compared to polyclonally-activated CD4+ T cells, S. aureus-specific Tcon were enriched for the expression of the Th17-type cytokine genes IL17A, IL22 and IL26, while higher percentages of S. aureus-specific Treg expressed the T Cell Immunoreceptor with Ig and ITIM domains (TIGIT), a pleiotropic immune checkpoint. Notably, the antagonistic anti-TIGIT mAb Tiragolumab increased IL-1β production in response to S. aureus in vitro. Therefore, these results uncover the presence of S. aureus-specific TIGIT+ Treg in the blood of healthy subjects that could blunt responses to vaccination and indicate TIGIT as a potential targetable biomarker to overcome pre-exposure-induced immunosuppression.
Copyright © 2025 Clegg, Mnich, Carignano, Cova, Tavarini, Sammicheli, Clemente, Smith, Siena, Bardelli, Brazzoli, Bagnoli, McLoughlin and Soldaini.
-
Cardiovascular biology
-
Immunology and Microbiology
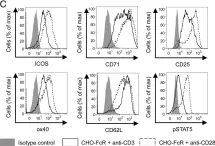
In Front Immunol on 10 April 2021 by Muller, Y. D., Nguyen, D. P., et al.
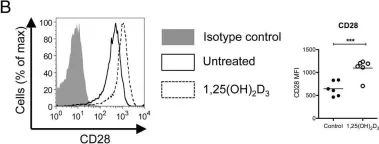
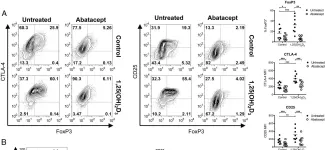
Fig.2.A

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 11
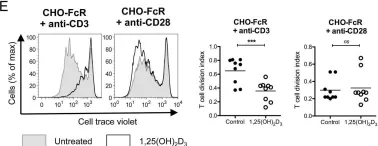
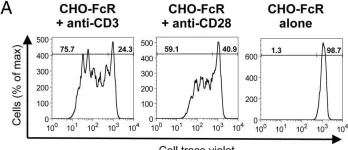
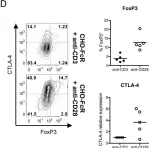
In Front Immunol on 10 April 2021 by Muller, Y. D., Nguyen, D. P., et al.
Fig.3.B

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 11
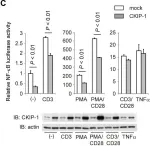
In Sci Rep on 27 November 2017 by Tello-Lafoz, M., Rodríguez-Rodríguez, C., et al.
Fig.8.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 11
In J Immunol on 15 September 2015 by Gardner, D. H., Jeffery, L. E., et al.
Fig.1.A

-
FC/FACS
-
Collected and cropped from J Immunol by CiteAb, provided under a CC-BY license
Image 1 of 11
In J Immunol on 15 September 2015 by Gardner, D. H., Jeffery, L. E., et al.
Fig.4.B

-
FC/FACS
-
Collected and cropped from J Immunol by CiteAb, provided under a CC-BY license
Image 1 of 11
In J Immunol on 15 September 2015 by Gardner, D. H., Jeffery, L. E., et al.
Fig.4.A

-
FC/FACS
-
Collected and cropped from J Immunol by CiteAb, provided under a CC-BY license
Image 1 of 11
In J Immunol on 15 September 2015 by Gardner, D. H., Jeffery, L. E., et al.
Fig.5.E

-
FC/FACS
-
Collected and cropped from J Immunol by CiteAb, provided under a CC-BY license
Image 1 of 11
In J Immunol on 15 September 2015 by Gardner, D. H., Jeffery, L. E., et al.
Fig.5.A

-
FC/FACS
-
Collected and cropped from J Immunol by CiteAb, provided under a CC-BY license
Image 1 of 11
In J Immunol on 15 September 2015 by Gardner, D. H., Jeffery, L. E., et al.
Fig.5.C

-
FC/FACS
-
Collected and cropped from J Immunol by CiteAb, provided under a CC-BY license
Image 1 of 11
In J Immunol on 15 September 2015 by Gardner, D. H., Jeffery, L. E., et al.
Fig.5.D

-
FC/FACS
-
Collected and cropped from J Immunol by CiteAb, provided under a CC-BY license
Image 1 of 11
In PLoS One on 28 January 2014 by Sakamoto, T., Kobayashi, M., et al.
Fig.1.C

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 11