Hypertrophic scar (HS) is a common fibroproliferative disorders with no fully effective treatments. The conversion of fibroblasts to myofibroblasts is known to play a critical role in HS formation, making it essential to identify molecules that promote myofibroblast dedifferentiation and to elucidate their underlying mechanisms. In this study, we used comparative transcriptomics and single-cell sequencing to identify key molecules and pathways that mediate fibrosis and myofibroblast transdifferentiation. Epidermal stem cell-derived extracellular vesicles (EpiSC-EVs) were isolated via ultracentrifugation and filtration, followed by miRNA sequencing to identify miRNAs targeting key molecules. After in vitro and in vivo treatment with EpiSC-EVs, we assessed antifibrotic effects through scratch assays, collagen contraction assays, Western blotting, and immunofluorescence. Transcriptomic sequencing and rescue experiments were used to investigate the molecular mechanism by which miR-203a-3p in EpiSC-EVs induces myofibroblast dedifferentiation. Our results indicate that PIK3CA is overexpressed in HS tissues and positively correlates with fibrosis. EpiSC-EVs were absorbed by scar-derived fibroblasts, promoting dedifferentiation from myofibroblasts to quiescent fibroblasts. Mechanistically, miR-203a-3p in EpiSC-EVs plays an essential role in inhibiting PIK3CA expression and PI3K/AKT/mTOR pathway hyperactivation, thereby reducing scar formation. In vivo studies confirmed that EpiSC-EVs attenuate excessive scarring through the miR-203a-3p/PIK3CA axis, suggesting EpiSC-EVs as a promising therapeutic approach for HS.
© 2025. The Author(s).
Product Citations: 145
In Journal of Nanobiotechnology on 29 January 2025 by Zhao, S., Kong, H., et al.
-
FC/FACS
-
Stem Cells and Developmental Biology
Preprint on BioRxiv : the Preprint Server for Biology on 22 January 2025 by Ishikawa, M., Ngo, Y. X., et al.
Summary Epithelial stem cells exhibit heterogeneity, with distinct stem cell populations occupying specific tissue regions. The human skin displays a characteristic undulating structure at the epidermal-dermal junction, which supports mechanical strength and influences the spatial organization of epithelial stem cells. Unlike human skin, mouse skin lacks these undulations, complicating studies into the effects of tissue architecture on stem cell distribution. Here, we leverage the mouse oral mucosa, which possesses an undulating structure similar to human skin, to characterize stem cell division dynamics and long-term fate in vivo . Using a combination of H2B-GFP pulse-chase analysis and lineage tracing with Dlx1-CreER and Slc1a3-CreER models, we demonstrate that slow-and fast-cycling stem cells localize to distinct anatomical regions relative to the undulating structure and maintain their respective compartments during tissue homeostasis. A three-dimensional culture model using micropatterned collagen scaffolds that recapitulate the undulating structures in vitro reveals that the mechanical environment generated by the undulating structures partially induces proliferative heterogeneity in epithelial stem cells. This study proposes tissue undulating surface structure as a common principle as a niche component that defines the localization of compartmentalized stem cell populations across different epithelial tissues. Abstract Figure
-
Stem Cells and Developmental Biology
In Cell Death & Disease on 28 July 2024 by Pinelli, M., Makdissi, S., et al.
Intestinal epithelial cells line the luminal surface to establish the intestinal barrier, where the cells play essential roles in the digestion of food, absorption of nutrients and water, protection from microbial infections, and maintaining symbiotic interactions with the commensal microbial populations. Maintaining and coordinating all these functions requires tight regulatory signaling, which is essential for intestinal homeostasis and organismal health. Dysfunction of intestinal epithelial cells, indeed, is linked to gastrointestinal disorders such as irritable bowel syndrome, inflammatory bowel disease, and gluten-related enteropathies. Emerging evidence suggests that peroxisome metabolic functions are crucial in maintaining intestinal epithelial cell functions and intestinal epithelium regeneration and, therefore, homeostasis. Here, we investigated the molecular mechanisms by which peroxisome metabolism impacts enteric health using the fruit fly Drosophila melanogaster and murine model organisms and clinical samples. We show that peroxisomes control cellular cholesterol, which in turn regulates the conserved yes-associated protein-signaling and contributes to intestinal epithelial structure and epithelial barrier function. Moreover, analysis of intestinal organoid cultures derived from biopsies of patients affected by Crohn's Disease revealed that the dysregulation of peroxisome number, excessive cellular cholesterol, and inhibition of Yap-signaling are markers of disease and could be novel diagnostic and/or therapeutic targets for treating Crohn's Disease. Our studies provided mechanistic insights on peroxisomal signaling in intestinal epithelial cell functions and identified cholesterol as a novel metabolic regulator of yes-associated protein-signaling in tissue homeostasis.
© 2024. The Author(s).
-
Biochemistry and Molecular biology
-
Cell Biology
In Nature Communications on 27 January 2024 by An, L., Kim, D., et al.
Vitiligo is an autoimmune skin disease caused by cutaneous melanocyte loss. Although phototherapy and T cell suppression therapy have been widely used to induce epidermal re-pigmentation, full pigmentation recovery is rarely achieved due to our poor understanding of the cellular and molecular mechanisms governing this process. Here, we identify unique melanocyte stem cell (McSC) epidermal migration rates between male and female mice, which is due to sexually dimorphic cutaneous inflammatory responses generated by ultra-violet B exposure. Using genetically engineered mouse models, and unbiased bulk and single-cell mRNA sequencing approaches, we determine that manipulating the inflammatory response through cyclooxygenase and its downstream prostaglandin product regulates McSC proliferation and epidermal migration in response to UVB exposure. Furthermore, we demonstrate that a combinational therapy that manipulates both macrophages and T cells (or innate and adaptive immunity) significantly promotes epidermal melanocyte re-population. With these findings, we propose a novel therapeutic strategy for repigmentation in patients with depigmentation conditions such as vitiligo.
© 2024. The Author(s).
-
Mus musculus (House mouse)
-
Stem Cells and Developmental Biology
In Cell Communication and Signaling : CCS on 19 January 2024 by Karbanová, J., Deniz, I. A., et al.
The incidence of melanoma is increasing worldwide. Since metastatic melanoma is highly aggressive, it is important to decipher all the biological aspects of melanoma cells. In this context, we have previously shown that metastatic FEMX-I melanoma cells release small (< 150 nm) extracellular vesicles (EVs) known as exosomes and ectosomes containing the stem (and cancer stem) cell antigenic marker CD133. EVs play an important role in intercellular communication, which could have a micro-environmental impact on surrounding tissues.
We report here a new type of large CD133+ EVs released by FEMX-I cells. Their sizes range from 2 to 6 µm and they contain lipid droplets and mitochondria. Real-time video microscopy revealed that these EVs originate from the lipid droplet-enriched cell extremities that did not completely retract during the cell division process. Once released, they can be taken up by other cells. Silencing CD133 significantly affected the cellular distribution of lipid droplets, with a re-localization around the nuclear compartment. As a result, the formation of large EVs containing lipid droplets was severely compromised.
Given the biochemical effect of lipid droplets and mitochondria and/or their complexes on cell metabolism, the release and uptake of these new large CD133+ EVs from dividing aggressive melanoma cells can influence both donor and recipient cells, and therefore impact melanoma growth and dissemination.
© 2024. The Author(s).
-
Homo sapiens (Human)
-
Cancer Research
-
Cell Biology
-
Endocrinology and Physiology
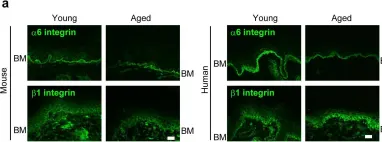
In Elife on 11 July 2017 by Watanabe, M., Natsuga, K., et al.
Fig.4.A

-
IHC-IF
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 2
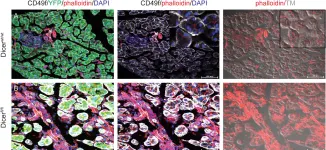
In PLoS One on 19 November 2014 by Wang, Y. J., McAllister, F., et al.
Fig.2.A,B

-
IHC
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 2