Intra-tumoral heterogeneity poses a major challenge to treating and managing cancer patients. A characteristic feature of melanoma is its composition of cancer cells with typically heterogeneous content of melanin pigment, the production of which is a hallmark of normal melanocytic differentiation but of poorly understood consequence in melanoma cells, as prospective assessment of pigment heterogeneity in melanoma cells has been experimentally challenging. Here, we describe a novel flow cytometric method for high purity separation of viable melanoma cells based on their melanin content, exploiting the light scattering properties of melanin. By fluorescence-activated cell sorting, we show that cells with low-pigment content (LPCs) in melanoma cell lines and patient tumors are usually far more abundant than high-pigment cells (HPCs) and have substantially increased potentials for colony formation in vitro and tumor formation in vivo. In RNAseq analysis, HPCs showed P53 activation and perturbed cell cycling, whereas LPCs displayed upregulation of MYC-associated transcription and activated ribosome biogenesis. In proof-of-concept studies, the latter was targeted by topoisomerase 2 beta targeting with CX-5461, which induced senescent HPC phenotypes and irreversible loss of clonogenic activity. These data indicate an 'inverted pyramid' hierarchical model of melanoma cell propagation wherein abundant LPCs frequently renew their own malignant potential to propagate disease but also infrequently generate HPCs that spontaneously lose this ability in a manner that might be exploited as an anti-melanoma strategy.
© 2025 The Author(s). Pigment Cell & Melanoma Research published by John Wiley & Sons Ltd.
Product Citations: 62
In Pigment Cell Melanoma Research on 1 July 2025 by Fedele, C., Kuser Abali, G., et al.
-
Cancer Research
Preprint on BioRxiv : the Preprint Server for Biology on 20 March 2025 by Romano, M., Musicò, A., et al.
Triple-negative breast cancer is an aggressive breast cancer subtype characterized by the absence of human epidermal growth factor receptor 2, estrogen and progesterone receptors, limiting targeted therapy options. Cisplatin, a chemotherapeutic agent, induces DNA damage and exhibits some efficacy against triple-negative breast cancer, but its effectiveness is often reduced by chemoresistance and systemic toxicity. A very promising strategy to augment cisplatin treatment can be based on combining it with the biologic Cetuximab, an epidermal growth factor receptor inhibitor, which boosts cisplatin efficacy by inducing ferroptosis. To optimize this synergy in a biocompatible and precise manner, we developed a nanoplatform based on red blood cell-derived extracellular vesicles for the synergistic delivery of Cetuximab and cisplatin. This formulation increases cisplatin uptake, as demonstrated in vitro and in patient-derived organoids, effectively reduces chemoresistance by downregulating hypoxia-related genes and upregulating ferroptosis-associated genes, and enhances cisplatin cytotoxicity while mitigating hemotoxicity compared to free cisplatin administration.
-
Cancer Research
-
Cardiovascular biology
R-loop functions in Brca1-associated mammary tumorigenesis.
In Proceedings of the National Academy of Sciences of the United States of America on 13 August 2024 by Chiang, H. C., Qi, L., et al.
Deleterious accumulation of R-loops, a DNA-RNA hybrid structure, contributes to genome instability. They are associated with BRCA1 mutation-related breast cancer, an estrogen receptor α negative (ERα-) tumor type originating from luminal progenitor cells. However, a presumed causality of R-loops in tumorigenesis has not been established in vivo. Here, we overexpress mouse Rnaseh1 (Rh1-OE) in vivo to remove accumulated R-loops in Brca1-deficient mouse mammary epithelium (BKO). R-loop removal exacerbates DNA replication stress in proliferating BKO mammary epithelial cells, with little effect on homology-directed repair of double-strand breaks following ionizing radiation. Compared to their BKO counterparts, BKO-Rh1-OE mammary glands contain fewer luminal progenitor cells but more mature luminal cells. Despite a similar incidence of spontaneous mammary tumors in BKO and BKO-Rh1-OE mice, a significant percentage of BKO-Rh1-OE tumors express ERα and progesterone receptor. Our results suggest that rather than directly elevating the overall tumor incidence, R-loops influence the mammary tumor subtype by shaping the cell of origin for Brca1 tumors.
IGF1R signaling induces epithelial-mesenchymal plasticity via ITGAV in cutaneous carcinoma.
In Journal of Experimental & Clinical Cancer Research : CR on 29 July 2024 by Lopez-Cerda, M., Lorenzo-Sanz, L., et al.
Early cutaneous squamous cell carcinomas (cSCCs) generally show epithelial differentiation features and good prognosis, whereas advanced cSCCs present mesenchymal traits associated with tumor relapse, metastasis, and poor survival. Currently, the mechanisms involved in cSCC progression are unclear, and the established markers are suboptimal for accurately predicting the clinical course of the disease.
Using a mouse model of cSCC progression, expression microarray analysis, immunofluorescence and flow cytometry assays, we have identified a prognostic biomarker of tumor relapse, which has been evaluated in a cohort of cSCC patient samples. Phosphoproteomic analysis have revealed signaling pathways induced in epithelial plastic cancer cells that promote epithelial-mesenchymal plasticity (EMP) and tumor progression. These pathways have been validated by genetic and pharmacological inhibition assays.
We show that the emergence of epithelial cancer cells expressing integrin αV (ITGAV) promotes cSCC progression to a mesenchymal state. Consistently, ITGAV expression allows the identification of patients at risk of cSCC relapse above the currently employed clinical histopathological parameters. We also demonstrate that activation of insulin-like growth factor-1 receptor (IGF1R) pathway in epithelial cancer cells is necessary to induce EMP and mesenchymal state acquisition in response to tumor microenvironment-derived factors, while promoting ITGAV expression. Likewise, ITGAV knockdown in epithelial plastic cancer cells also blocks EMP acquisition, generating epithelial tumors.
Our results demonstrate that ITGAV is a prognostic biomarker of relapse in cSCCs that would allow improved patient stratification. ITGAV also collaborates with IGF1R to induce EMP in epithelial cancer cells and promotes cSCC progression, revealing a potential therapeutic strategy to block the generation of advanced mesenchymal cSCCs.
© 2024. The Author(s).
-
FC/FACS
-
Cancer Research
Breast cancer patient-derived organoids for the investigation of patient-specific tumour evolution.
In Cancer Cell International on 27 June 2024 by Mazzucchelli, S., Signati, L., et al.
A reliable preclinical model of patient-derived organoids (PDOs) was developed in a case study of a 69-year-old woman diagnosed with breast cancer (BC) to investigate the tumour evolution before and after neoadjuvant chemotherapy and surgery. The results were achieved due to the development of PDOs from tissues collected before (O-PRE) and after (O-POST) treatment.
PDO cultures were characterized by histology, immunohistochemistry (IHC), transmission electron microscopy (TEM), scanning electron microscopy (SEM), confocal microscopy, flow cytometry, real-time PCR, bulk RNA-seq, single-cell RNA sequencing (scRNA-seq) and drug screening.
Both PDO cultures recapitulated the histological and molecular profiles of the original tissues, and they showed typical mammary gland organization, confirming their reliability as a personalized in vitro model. Compared with O-PRE, O-POST had a greater proliferation rate with a significant increase in the Ki67 proliferation index. Moreover O-POST exhibited a more stem-like and aggressive phenotype, with increases in the CD24low/CD44low and EPCAMlow/CD49fhigh cell populations characterized by increased tumour initiation potential and multipotency and metastatic potential in invasive lobular carcinoma. Analysis of ErbB receptor expression indicated a decrease in HER-2 expression coupled with an increase in EGFR expression in O-POST. In this context, deregulation of the PI3K/Akt signalling pathway was assessed by transcriptomic analysis, confirming the altered transcriptional profile. Finally, transcriptomic single-cell analysis identified 11 cell type clusters, highlighting the selection of the luminal component and the decrease in the number of Epithelial-mesenchymal transition cell types in O-POST.
Neoadjuvant treatment contributed to the enrichment of cell populations with luminal phenotypes that were more resistant to chemotherapy in O-POST. PDOs represent an excellent 3D cell model for assessing disease evolution.
© 2024. The Author(s).
-
FC/FACS
-
Homo sapiens (Human)
-
Cancer Research
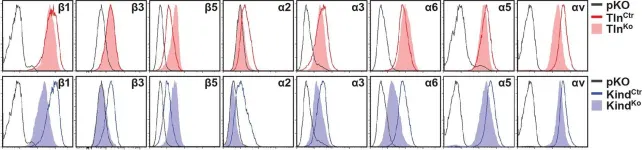
In Elife on 27 January 2016 by Theodosiou, M., Widmaier, M., et al.
Fig.2.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 6
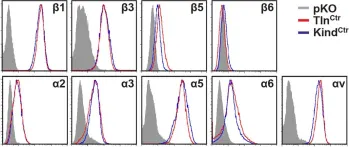
In Elife on 27 January 2016 by Theodosiou, M., Widmaier, M., et al.
Fig.1.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 6
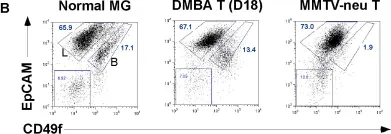
In PLoS One on 22 February 2012 by Kim, S., Roopra, A., et al.
Fig.3.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 6
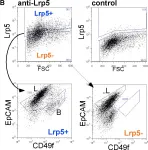
In PLoS One on 5 May 2011 by Kim, S., Goel, S., et al.
Fig.2.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 6
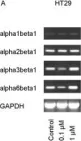
In BMC Cancer on 11 January 2005 by Engl, T., Makarević, J., et al.
Fig.6.A

-
WB
-
Collected and cropped from BMC Cancer by CiteAb, provided under a CC-BY license
Image 1 of 6
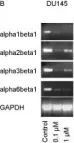
In BMC Cancer on 11 January 2005 by Engl, T., Makarević, J., et al.
Fig.6.B

-
WB
-
Collected and cropped from BMC Cancer by CiteAb, provided under a CC-BY license
Image 1 of 6