Although abnormal activities across multiple cell types are believed to contribute to the development of various neurodevelopmental disorders, current brain organoid technologies fall short in accurately modeling the dynamic cellular interactions in the human brain. Recently, we developed a cellular reconstitution technology to create human forebrain assembloids with enhanced cellular diversity, representing dynamic interactions between neurons and glial cells. Here, we created patient-derived, mix-and-match forebrain assembloids, in which neurons, astrocytes, and microglia from both healthy individuals and schizophrenia patients were reconstituted in a combinatorial manner, and identified aberrant cellular interactions between neurons and glial cells in human schizophrenia. At the early stage, schizophrenia forebrain assembloids showed premature neurogenesis induced by the abnormal proliferation and differentiation of neural progenitor cells. Integrated modular analysis of gene expression in post-mortem schizophrenia brain tissue and brain assembloids found increased expression of tumor protein p53 (TP53) and nuclear factor of activated T-cells 4 (NFATC4), which functioned as master transcriptional regulators to epigenetically reprogram the transcriptome involved in the cellular dynamics of neuronal progenitor cells, leading to premature neurogenesis. At the later stage, we observed weakened structures of laminar organization of the cortical layers in forebrain assembloids and identified the neuron-dependent transcriptional plasticity of glial cells and their altered signaling feedback with neurons, in which neuronal urocortin (UCN) and protein tyrosine phosphatase receptor type F (PTPRF) elicited the expression of Wnt family member 11 (WNT11) and thrombospondin 4 (THBS4) in astrocytes and microglia, respectively. These aberrant signaling axes altered the neuronal transcriptome associated with neuronal response to various stimuli and synthetic processes of biomolecules, resulting in reduced synapse connectivity. Thus, we elucidated developmental stage-specific, multifactorial mechanisms by which dynamic cellular interplay among neural progenitor cells, neurons, and glial cells contribute to the development of the human schizophrenia brain. Our study further demonstrated the potential and applicability of patient-derived forebrain assembloid technology to advance our understanding of the pathogenesis of various neurodevelopmental disorders.
Product Citations: 105
Preprint on BioRxiv : the Preprint Server for Biology on 23 December 2024 by Kim, E., Kim, Y., et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Neuroscience
Blood-brain barrier permeability increases with the differentiation of glioblastoma cells in vitro.
In Fluids and Barriers of the CNS on 1 November 2024 by Digiovanni, S., Lorenzati, M., et al.
Glioblastoma multiforme (GBM) is an aggressive tumor, difficult to treat pharmacologically because of the blood-brain barrier (BBB), which is rich in ATP-binding cassette (ABC) transporters and tight junction (TJ) proteins. The BBB is disrupted within GBM bulk, but it is competent in brain-adjacent-to-tumor areas, where eventual GBM foci can trigger tumor relapse. How GBM cells influence the permeability of BBB is poorly investigated.
To clarify this point, we co-cultured human BBB models with 3 patient-derived GBM cells, after separating from each tumor the stem cell/neurosphere (SC/NS) and the differentiated/adherent cell (AC) components. Also, we set up cultures of BBB cells with the conditioned medium of NS or AC, enriched or depleted of IL-6. Extracellular cytokines were measured by protein arrays and ELISA. The intracellular signaling in BBB cells was measured by immunoblotting, in the presence of STAT3 pharmacological inhibitor or specific PROTAC. The competence of BBB was evaluated by permeability assays and TEER measurement.
The presence of GBM cells or their conditioned medium increased the permeability to doxorubicin, mitoxantrone and dextran-70, decreased TEER, down-regulated ABC transporters and TJ proteins at the transcriptional level. These effects were higher with AC or their medium than with NS. The secretome analysis identified IL-6 as significantly more produced by AC than by NS. Notably, AC-conditioned medium treated with an IL-6 neutralizing antibody reduced the BBB permeability to NS levels, while NS-conditioned medium enriched with IL-6 increased BBB permeability to AC levels. Mechanistically, IL-6 released by AC GBM cells activated STAT3 in BBB cells. In turn, STAT3 down-regulated ABC transporter and TJ expression, increased permeability and decreased TEER. The same effects were obtained in BBB cells treated with STA-21, a pharmacological inhibitor of STAT3, or with a PROTAC targeting STAT3.
Our work demonstrates for the first time that the degree of GBM differentiation influences BBB permeability. The crosstalk between GBM cells that release IL-6 and BBB cells that respond by activating STAT3, controls the expression of ABC transporters and TJ proteins on BBB. These results may pave the way for novel therapeutic tools to tune BBB permeability and improve drug delivery to GBM.
© 2024. The Author(s).
-
Cardiovascular biology
In Frontiers in Physiology on 1 October 2024 by Olczak, A., Pieczonka, T. D., et al.
Mice hair follicles (HFs) are a valuable model for studying various aspects of hair biology, including morphogenesis, development, and regeneration due to their easily observable phenotype and genetic manipulability. The initiation and progression of hair follicle morphogenesis, as well as the hair follicle cycle, are regulated by various signaling pathways, of which the main role is played by the Wingless-type MMTV integration site family (Wnt) and the Bone Morphogenic Protein (BMP). During the hair follicle cycle, the BMP pathway maintains hair follicle stem cells (HFSCs) in a dormant state while the Wnt pathway activates them for hair growth. Given the pivotal role of the Wnt pathway in hair biology and HFSCs regulation, we investigated the influence of the Wnt modulator - R-spondin 3 (Rspo3), in these processes. For this purpose, we developed a transgenic mice model with the overexpression of Rspo3 (Rspo3GOF) in the whole ectoderm and its derivatives, starting from early morphogenesis. Rspo3GOF mice exhibited a distinct phenotype with sparse hair and visible bald areas, caused by reduced proliferation and increased apoptosis of hair matrix progenitor cells, which resulted in a premature anagen-to-catagen transition with a shortened growth phase and decreased overall length of all hair types. In addition, Rspo3GOF promoted induction of auchene and awl, canonical Wnt-dependent hair type during morphogenesis, but the overall hair amount remained reduced. We also discovered a delay in the pre-bulge formation during morphogenesis and prolonged immaturity of the HFSC population in the bulge region postnatally, which further impaired proper hair regeneration throughout the mice's lifespan. Our data supported that Rspo3 function observed in our model works in HFSCs' formation of pre-bulge during morphogenesis via enhancing activation of the canonical Wnt pathway, whereas in contrast, in the postnatal immature bulge, activation of canonical Wnt signaling was attenuated. In vitro studies on keratinocytes revealed changes in proliferation, migration, and colony formation, highlighting the inhibitory effect of constitutive overexpression of Rspo3 on these cellular processes. Our research provides novel insights into the role of Rspo3 in the regulation of hair morphogenesis and development, along with the formation and maturation of the HFSCs, which affect hair regeneration.
Copyright © 2024 Olczak, Pieczonka, Ławicki, Łukaszyk, Pulawska-Czub, Cambier and Kobielak.
-
Mus musculus (House mouse)
-
Endocrinology and Physiology
-
Stem Cells and Developmental Biology
In Frontiers in Immunology on 1 October 2024 by Govaerts, J., Van Breedam, E., et al.
Varicella-zoster virus (VZV) encephalitis and meningitis are potential central nervous system (CNS) complications following primary VZV infection or reactivation. With Type-I interferon (IFN) signalling being an important first line cellular defence mechanism against VZV infection by the peripheral tissues, we here investigated the triggering of innate immune responses in a human neural-like environment. For this, we established and characterised 5-month matured hiPSC-derived neurospheroids (NSPHs) containing neurons and astrocytes. Subsequently, NSPHs were infected with reporter strains of VZV (VZVeGFP-ORF23) or Sendai virus (SeVeGFP), with the latter serving as an immune-activating positive control. Live cell and immunocytochemical analyses demonstrated VZVeGFP-ORF23 infection throughout the NSPHs, while SeVeGFP infection was limited to the outer NSPH border. Next, NanoString digital transcriptomics revealed that SeVeGFP-infected NSPHs activated a clear Type-I IFN response, while this was not the case in VZVeGFP-ORF23-infected NSPHs. Moreover, the latter displayed a strong suppression of genes related to IFN signalling and antigen presentation, as further demonstrated by suppression of IL-6 and CXCL10 production, failure to upregulate Type-I IFN activated anti-viral proteins (Mx1, IFIT2 and ISG15), as well as reduced expression of CD74, a key-protein in the MHC class II antigen presentation pathway. Finally, even though VZVeGFP-ORF23-infection seems to be immunologically ignored in NSPHs, its presence does result in the formation of stress granules upon long-term infection, as well as disruption of cellular integrity within the infected NSPHs. Concluding, in this study we demonstrate that 5-month matured hiPSC-derived NSPHs display functional innate immune reactivity towards SeV infection, and have the capacity to recapitulate the strong immune evasive behaviour towards VZV.
Copyright © 2024 Govaerts, Van Breedam, De Beuckeleer, Goethals, D’Incal, Di Stefano, Van Calster, Buyle-Huybrecht, Boeren, De Reu, Paludan, Thiry, Lebrun, Sadzot-Delvaux, Motaln, Rogelj, Van Weyenbergh, De Vos, Vanden Berghe, Ogunjimi, Delputte and Ponsaerts.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
-
Neuroscience
In Nature Communications on 11 July 2024 by Neumayer, G., Torkelson, J. L., et al.
We present Dystrophic Epidermolysis Bullosa Cell Therapy (DEBCT), a scalable platform producing autologous organotypic iPS cell-derived induced skin composite (iSC) grafts for definitive treatment. Clinical-grade manufacturing integrates CRISPR-mediated genetic correction with reprogramming into one step, accelerating derivation of COL7A1-edited iPS cells from patients. Differentiation into epidermal, dermal and melanocyte progenitors is followed by CD49f-enrichment, minimizing maturation heterogeneity. Mouse xenografting of iSCs from four patients with different mutations demonstrates disease modifying activity at 1 month. Next-generation sequencing, biodistribution and tumorigenicity assays establish a favorable safety profile at 1-9 months. Single cell transcriptomics reveals that iSCs are composed of the major skin cell lineages and include prominent holoclone stem cell-like signatures of keratinocytes, and the recently described Gibbin-dependent signature of fibroblasts. The latter correlates with enhanced graftability of iSCs. In conclusion, DEBCT overcomes manufacturing and safety roadblocks and establishes a reproducible, safe, and cGMP-compatible therapeutic approach to heal lesions of DEB patients.
© 2024. The Author(s).
-
FC/FACS
In J Cell Mol Med on 1 October 2020 by Ding, Y., Gao, S., et al.
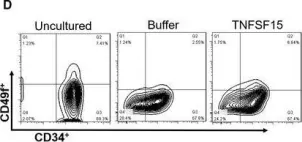
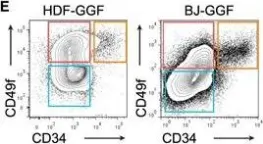
Fig.1.D
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from J Cell Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 5
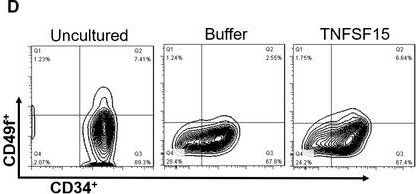
In J Cell Mol Med on 1 October 2020 by Ding, Y., Gao, S., et al.
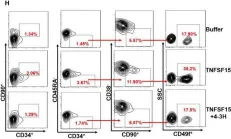
Fig.1.H
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from J Cell Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 5
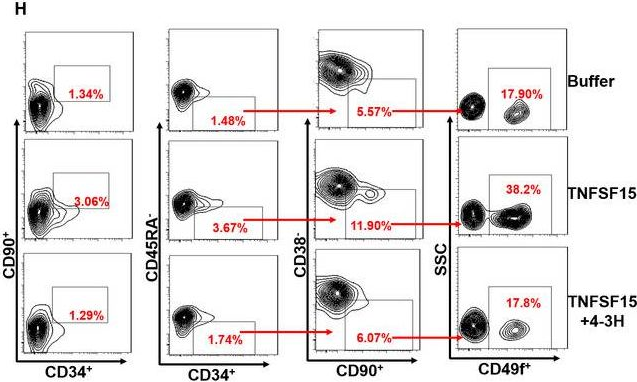
In J Cell Mol Med on 1 October 2020 by Ding, Y., Gao, S., et al.
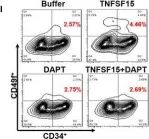
Fig.5.L
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from J Cell Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 5
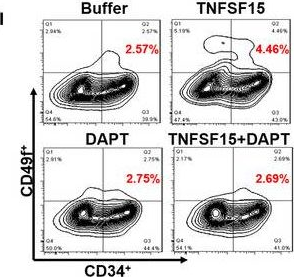
In Cell Rep on 4 December 2018 by Gomes, A. M., Kurochkin, I., et al.
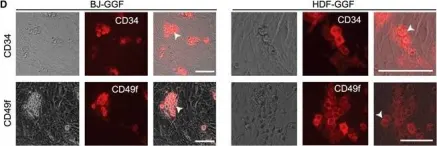
Fig.1.D

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Cell Rep by CiteAb, provided under a CC-BY license
Image 1 of 5
In Cell Rep on 4 December 2018 by Gomes, A. M., Kurochkin, I., et al.
Fig.1.E

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Cell Rep by CiteAb, provided under a CC-BY license
Image 1 of 5