Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccines are effective at protecting from severe disease, but the protective antibodies wane rapidly even though SARS-CoV-2-specific plasma cells can be found in the bone marrow (BM). Here, to explore this paradox, we enrolled 19 healthy adults at 2.5-33 months after receipt of a SARS-CoV-2 mRNA vaccine and measured influenza-, tetanus- or SARS-CoV-2-specific antibody-secreting cells (ASCs) in long-lived plasma cell (LLPC) and non-LLPC subsets within the BM. Only influenza- and tetanus-specific ASCs were readily detected in the LLPCs, whereas SARS-CoV-2 specificities were mostly absent. The ratios of non-LLPC:LLPC for influenza, tetanus and SARS-CoV-2 were 0.61, 0.44 and 29.07, respectively. In five patients with known PCR-proven history of recent infection and vaccination, SARS-CoV-2-specific ASCs were mostly absent from the LLPCs. We show similar results with measurement for secreted antibodies from BM ASC culture supernatant. While serum IgG titers specific for influenza and tetanus correlated with IgG LLPCs, serum IgG levels for SARS-CoV-2, which waned within 3-6 months after vaccination, were associated with IgG non-LLPCs. In all, our studies suggest that rapid waning of SARS-CoV-2-specific serum antibodies could be accounted for by the absence of BM LLPCs after these mRNA vaccines.
© 2024. The Author(s).
Product Citations: 91
In Nature Medicine on 1 January 2025 by Nguyen, D. C., Hentenaar, I. T., et al.
-
COVID-19
-
Genetics
In Methods in Molecular Biology (Clifton, N.J.) on 30 September 2024 by Pinto, T. N. C., Benard, G., et al.
B cells are crucial components of the immune system, responsible for producing specific antibodies in response to infections and vaccines. Despite their uniform appearance, B cells display diverse surface molecules and functional properties, which are not yet fully understood. Apart from antibody production, B cells also play roles in antigen presentation and cytokine secretion, essential for initiating T-cell immune responses. Their significance as disease biomarkers and therapeutic targets has led to increased research focus. However, the lack of standardized protocols for B-cell identification and the variability in defining B-lymphocyte subpopulations pose some challenges. This paper proposes a B-cell identification panel throughout the evaluation of previous cytometry panels and nomenclature heterogeneity for B-cell subpopulations. Major subpopulations recognized in human peripheral blood include transitional, naive, switched memory, unswitched memory, double negative, and plasmablasts, characterized based on their functional and phenotypic features. We present a standardized flow cytometry protocol utilizing surface phenotypic markers (CD3, CD19, IgD, CD27, CD38, and CD24) to differentiate and analyze B-cell subpopulations. This practical and cost-effective panel can be used in various research and laboratory settings. The challenges of standardizing names and markers for classifying B-lymphocyte subpopulations are discussed, along with protocols utilizing multiple markers and gating strategies, allied with the importance of considering viability markers. In summary, this standardized protocol and panel provide a comprehensive approach to identifying B-cell subpopulations to enhance the reproducibility and comparability of B-cell subpopulation studies.
© 2025. The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature.
-
Biochemistry and Molecular biology
-
Cardiovascular biology
In Front Aging on 9 September 2024 by Frasca, D. & Bueno, V.
In this paper, we measured B cell function in elderly healthy individuals (EH) and in elderly patients with Type-2 Diabetes Mellitus (T2DM, ET2DM), which are treatment-naive, as compared to healthy young (YH) individuals. Results show a higher serum inflammatory status of elderly versus young individuals, and especially of ET2DM versus EH. This status is associated with a reduced response to the seasonal influenza vaccine and with increased frequencies of the circulating pro-inflammatory B cell subset called Double Negative (DN) B cells. B cells from ET2DM patients are not only more inflammatory but also hyper-metabolic as compared to those from EH controls. The results herein are to our knowledge the first to show that T2DM superimposed on aging further increases systemic and B cell intrinsic inflammation, as well as dysfunctional humoral immunity. Our findings confirm and extend our previously published findings showing that inflammatory B cells are metabolically supported.
Copyright © 2024 Frasca and Bueno.
-
FC/FACS
-
Cell Biology
-
Immunology and Microbiology
Immunometabolic effects of lactate on humoral immunity in healthy individuals of different ages.
In Nature Communications on 30 August 2024 by Romero, M., Miller, K., et al.
Aging is characterized by chronic systemic inflammation and metabolic changes. We compare the metabolic status of B cells from young and elderly donors and found that aging is associated with higher oxygen consumption rates, and especially higher extracellular acidification rates, measures of oxidative phosphorylation and of anaerobic glycolysis, respectively. Importantly, this higher metabolic status, which reflects age-associated expansion of pro-inflammatory B cells, is found associated with higher secretion of lactate and autoimmune antibodies after in vitro stimulation. B cells from elderly individuals induce in vitro polarization of CD4+ T cells from young individuals into pro-inflammatory CD4+ T cells through metabolic pathways mediated by lactate, which can be inhibited by targeting lactate enzymes and transporters, as well as signaling pathways supporting anaerobic glycolysis. Lactate also induces immunosenescent B cells that are glycolytic, express transcripts for multiple pro-inflammatory molecules, and are characterized by a higher metabolic status. These results altogether may have relevant clinical implications and suggest alternative targets for therapeutic interventions in the elderly and patients with inflammatory conditions and diseases.
© 2024. The Author(s).
-
Immunology and Microbiology
In Nature Communications on 15 June 2024 by Wang, Y., Hao, A., et al.
The Omicron subvariants BQ.1.1, XBB.1.5, and XBB.1.16 of SARS-CoV-2 are known for their adeptness at evading immune responses. Here, we isolate a neutralizing antibody, 7F3, with the capacity to neutralize all tested SARS-CoV-2 variants, including BQ.1.1, XBB.1.5, and XBB.1.16. 7F3 targets the receptor-binding motif (RBM) region and exhibits broad binding to a panel of 37 RBD mutant proteins. We develop the IgG-like bispecific antibody G7-Fc using 7F3 and the cross-neutralizing antibody GW01. G7-Fc demonstrates robust neutralizing activity against all 28 tested SARS-CoV-2 variants and sarbecoviruses, providing potent prophylaxis and therapeutic efficacy against XBB.1 infection in both K18-ACE and BALB/c female mice. Cryo-EM structure analysis of the G7-Fc in complex with the Omicron XBB spike (S) trimer reveals a trimer-dimer conformation, with G7-Fc synergistically targeting two distinct RBD epitopes and blocking ACE2 binding. Comparative analysis of 7F3 and LY-CoV1404 epitopes highlights a distinct and highly conserved epitope in the RBM region bound by 7F3, facilitating neutralization of the immune-evasive Omicron variant XBB.1.16. G7-Fc holds promise as a potential prophylactic countermeasure against SARS-CoV-2, particularly against circulating and emerging variants.
© 2024. The Author(s).
-
COVID-19
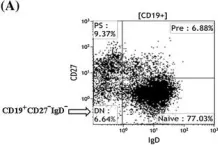
In Arthritis Res Ther on 14 March 2015 by Mahmood, Z., Muhammad, K., et al.
Fig.1.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Arthritis Res Ther by CiteAb, provided under a CC-BY license
Image 1 of 2
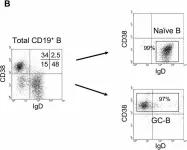
In BMC Immunol on 4 February 2005 by Kim, J. R., Lim, H. W., et al.
Fig.2.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from BMC Immunol by CiteAb, provided under a CC-BY license
Image 1 of 2