Multiple sclerosis (MS) is a prototypical autoimmune disease of the central nervous system (CNS). In addition to CD4+ T cells, memory B cells are now recognized as a critical cell type in the disease. This is underlined by the fact that the best-characterized environmental risk factor for MS is the Epstein-Barr virus (EBV), which can infect and persist in memory B cells throughout life. Several studies have identified changes in anti-EBV immunity in patients with MS. Examples include elevated titers of anti-EBV nuclear antigen 1 (EBNA1) antibodies, interactions of these with the MS-associated HLA-DR15 haplotype, and molecular mimicry with MS autoantigens like myelin basic protein (MBP), anoctamin-2 (ANO2), glial cell adhesion molecule (GlialCAM), and alpha-crystallin B (CRYAB). In this study, we employ a simple in vitro assay to examine the memory B cell antibody repertoire in MS patients and healthy controls. We replicate previous serological data from MS patients demonstrating an increased secretion of anti-EBNA1380-641 IgG in cell culture supernatants, as well as a positive correlation of these levels with autoantibodies against GlialCAM262-416 and ANO21-275. For EBNA1380-641 and ANO21-275, we provide additional evidence suggesting antibody cross-reactivity between the two targets. Further, we show that two efficacious MS treatments - natalizumab (NAT) and autologous hematopoietic stem cell transplantation (aHSCT) - are associated with distinct changes in the EBNA1-directed B cell response and that these alterations can be attributed to the unique mechanisms of action of these therapies. Using an in vitro system, our study confirms MS-associated changes in the anti-EBNA1 memory B cell response, EBNA1380-641 antibody cross-reactivity with ANO21-275, and reveals treatment-associated changes in the immunoglobulin repertoire in MS.
Copyright © 2024 Marti, Ruder, Thomas, Bronge, De La Parra Soto, Grönlund, Olsson and Martin.
Product Citations: 57
In Frontiers in Immunology on 11 September 2024 by Marti, Z., Ruder, J., et al.
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Science Immunology on 5 April 2024 by Grigoryan, L., Feng, Y., et al.
Vaccine adjuvants increase the breadth of serum antibody responses, but whether this is due to the generation of antigen-specific B cell clones with distinct specificities or the maturation of memory B cell clones that produce broadly cross-reactive antibodies is unknown. Here, we longitudinally analyzed immune responses in healthy adults after two-dose vaccination with either a virus-like particle COVID-19 vaccine (CoVLP), CoVLP adjuvanted with AS03 (CoVLP+AS03), or a messenger RNA vaccination (mRNA-1273). CoVLP+AS03 enhanced the magnitude and durability of circulating antibodies and antigen-specific CD4+ T cell and memory B cell responses. Antigen-specific CD4+ T cells in the CoVLP+AS03 group at day 42 correlated with antigen-specific memory B cells at 6 months. CoVLP+AS03 induced memory B cell responses, which accumulated somatic hypermutations over 6 months, resulting in enhanced neutralization breadth of monoclonal antibodies. Furthermore, the fraction of broadly neutralizing antibodies encoded by memory B cells increased between day 42 and 6 months. These results indicate that AS03 enhances the antigenic breadth of B cell memory at the clonal level and induces progressive maturation of the B cell response.
-
COVID-19
-
Immunology and Microbiology
Multi-omics analysis of mucosal and systemic immunity to SARS-CoV-2 after birth.
In Cell on 12 October 2023 by Wimmers, F., Burrell, A. R., et al.
The dynamics of immunity to infection in infants remain obscure. Here, we used a multi-omics approach to perform a longitudinal analysis of immunity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in infants and young children by analyzing blood samples and weekly nasal swabs collected before, during, and after infection with Omicron and non-Omicron variants. Infection stimulated robust antibody titers that, unlike in adults, showed no sign of decay for up to 300 days. Infants mounted a robust mucosal immune response characterized by inflammatory cytokines, interferon (IFN) α, and T helper (Th) 17 and neutrophil markers (interleukin [IL]-17, IL-8, and CXCL1). The immune response in blood was characterized by upregulation of activation markers on innate cells, no inflammatory cytokines, but several chemokines and IFNα. The latter correlated with viral load and expression of interferon-stimulated genes (ISGs) in myeloid cells measured by single-cell multi-omics. Together, these data provide a snapshot of immunity to infection during the initial weeks and months of life.
Copyright © 2023 Elsevier Inc. All rights reserved.
-
COVID-19
-
Immunology and Microbiology
B-cell targeting with anti-CD38 daratumumab: implications for differentiation and memory responses.
In Life Science Alliance on 1 September 2023 by Verhoeven, D., Grinwis, L., et al.
B cell-targeted therapies, such as CD20-targeting mAbs, deplete B cells but do not target the autoantibody-producing plasma cells (PCs). PC-targeting therapies such as daratumumab (anti-CD38) form an attractive approach to treat PC-mediated diseases. CD38 possesses enzymatic and receptor capabilities, which may impact a range of cellular processes including proliferation and differentiation. However, very little is known whether and how CD38 targeting affects B-cell differentiation, in particular for humans beyond cancer settings. Using in-depth in vitro B-cell differentiation assays and signaling pathway analysis, we show that CD38 targeting with daratumumab demonstrated a significant decrease in proliferation, differentiation, and IgG production upon T cell-dependent B-cell stimulation. We found no effect on T-cell activation or proliferation. Furthermore, we demonstrate that daratumumab attenuated the activation of NF-κB in B cells and the transcription of NF-κB-targeted genes. When culturing sorted B-cell subsets with daratumumab, the switched memory B-cell subset was primarily affected. Overall, these in vitro data elucidate novel non-depleting mechanisms by which daratumumab can disturb humoral immune responses. Affecting memory B cells, daratumumab may be used as a therapeutic approach in B cell-mediated diseases other than the currently targeted malignancies.
© 2023 Verhoeven et al.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Tuberculosis (Edinburgh, Scotland) on 1 May 2023 by Girma, T., Tsegaye, A., et al.
Mortality and morbidity from tuberculosis (TB) remain one of the most important public health issues. Although cell-mediated immunity is the main immune response against Mycobacterium tuberculosis (MTB), the role of B-cells during MTB infection and disease is unclear.
Peripheral blood mononuclear cells (PBMC) were isolated from treatment naïve Pulmonary TB patients (TB, n = 16), latent TB-infected participants (LTBI, n = 17), and healthy controls (HC, n = 19). PBMCs were stained with various fluorescently labeled antibodies to define B-cell subsets using multicolor flow cytometry.
Atypical memory B cells (CD19+CD27-CD21-) and circulating marginal zone B-cells (CD19+CD27+CD21+IgM+IgD+CD23-) were significantly higher in active TB when compared to LTBI and HC. CD5+ regulatory B cells (Breg, CD19+CD24hiCD38hiCD5+) and resting B-cells (CD19+CD27+CD21+) in Active TB patients were significantly lower compared to HC and LTBI. Overall, there were no differences in B cell percentages (CD19+), naïve B cells (CD19+CD27-CD21+), Breg (CD19+CD24hiCD38hi), and activated memory B cells (CD19+CD27+CD21-) among the three study groups.
These results indicated that multiple subsets of B cells were associated with TB infection and disease. It will be useful to examine these cell populations for their potential use as biomarkers for TB disease and LTBI.
Published by Elsevier Ltd.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
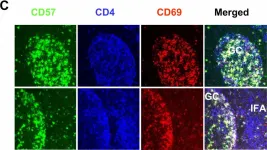
In BMC Immunol on 4 February 2005 by Kim, J. R., Lim, H. W., et al.
Fig.1.A

-
IHC-IF
-
Homo sapiens (Human)
Collected and cropped from BMC Immunol by CiteAb, provided under a CC-BY license
Image 1 of 2
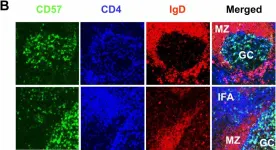
In BMC Immunol on 4 February 2005 by Kim, J. R., Lim, H. W., et al.
Fig.1.B

-
IHC-IF
-
Homo sapiens (Human)
Collected and cropped from BMC Immunol by CiteAb, provided under a CC-BY license
Image 1 of 2