Skin repair is a complex physiological process that involves the coordinated actions of various cell types. This study examines the distinct roles of amniotic mesenchymal stem cells (A-MSCs) and umbilical cord mesenchymal stem cells (UC-MSCs) in skin healing using a mouse model. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses revealed significant differences in gene expression between A-MSCs and UC-MSCs. Specifically, A-MSCs exhibited upregulation of genes associated with extracellular matrix (ECM) organisation and cell migration, thereby enhancing their tissue remodelling capabilities. In contrast, UC-MSCs demonstrate increased expression of genes involved in angiogenesis and anti-inflammatory responses, highlighting their role in creating a favourable healing environment. These findings highlight the unique therapeutic potentials of A-MSCs and UC-MSCs in skin repair strategies. Although MSCs hold promise in regenerative medicine, challenges such as optimal cell selection and modulation of the inflammatory microenvironment remain to be addressed. Our research emphasises the need for continued research related to properties of MSCs to refine therapeutic approaches for effective wound healing.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Product Citations: 109
Insights From Amniotic and Umbilical Cord Mesenchymal Stem Cells in Wound Healing.
In Journal of Cellular and Molecular Medicine on 1 June 2025 by Shen, N. E., Wu, Y., et al.
-
Biochemistry and Molecular biology
-
Stem Cells and Developmental Biology
In Frontiers in Immunology on 26 May 2025 by Bielski, P., Barczyński, J., et al.
The introduction of checkpoint immunotherapeutic agents in the last decade has revolutionized cancer treatment. Although anti-PD-1, anti-PD-L1 and anti-CTLA4 are promising therapies, many patients fail to respond or relapse due to drug resistance potentially due to redundancy of immune checkpoints. One of the ways to improve the efficacy of this cancer treatment is to target two or even three immune checkpoints. To date, the benefit of combined anti-VISTA/anti-PD-L1 therapy has been confirmed, but no one has investigated the efficacy of blocking these negative immune checkpoints with a bispecific anti-VISTA/anti-PD-L1 antibody.
In this study, the bispecific antibodies (bsAbs) were produced in three formats: symmetric (IgG-HC-scFv), asymmetric (Fab-scFv-Fc(KIH)) and 2 x scFv. The binding and blocking properties of these bispecific antibodies (bsAbs) and their efficacy compared to monotherapy and combination therapy were then determined using endometrial (RL95-2), pancreatic (PANC-1) and breast (BT-20) cancer cell lines.
The bsAbs generated in this study showed weaker binding properties to PD-1 and VISTA in ELISA (EC50) than the parent antibodies (atezolizumab and onvatilimab). Blockade of VISTA/VSIG-3 binding was also weaker with bsAbs compared to onvatilimab, but the ability to block the PD-1/PD-L1 pathway was slightly better than with atezolizumab. The Fc-based bsAbs showed statistically significant higher levels of lysis of endometrial, breast and pancreatic cancer cells. The symmetric bsAbs (IgG-HC-scFv) showed the most promising therapeutic potential. Higher levels of cancer cell lysis were associated with higher levels of pro-inflammatory cytokines. Both the asymmetric and symmetric bsAbs resulted in higher secretion levels of IFN-γ, TNFα and Granzyme B than anti-VISTA, anti-PD-L1 monotherapy and anti-VISTA/anti-PD-L1 combination therapy.
The high level of tumor cell lysis and increased expression of pro-inflammatory cytokines induced by the Fc-based bsAbs suggest a novel approach for the treatment of pancreatic, endometrial and breast cancer.
Copyright © 2025 Bielski, Barczyński, Mikitiuk, Myrcha, Rykała, Boon, Gąsior, Hec-Gałązka, Holak and Sitar.
-
Cancer Research
-
Immunology and Microbiology
In Stem Cell Reviews and Reports on 1 October 2024 by Tollance, A., Prola, A., et al.
Stem cell therapy holds significant potential for skeletal muscle repair, with in vitro-generated human muscle reserve cells (MuRCs) emerging as a source of quiescent myogenic stem cells that can be injected to enhance muscle regeneration. However, the clinical translation of such therapies is hampered by the need for fetal bovine serum (FBS) during the in vitro generation of human MuRCs. This study aimed to determine whether fresh allogeneic human platelet-rich plasma (PRP) combined or not with hyaluronic acid (PRP-HA) could effectively replace xenogeneic FBS for the ex vivo expansion and differentiation of human primary myoblasts. Cells were cultured in media supplemented with either PRP or PRP-HA and their proliferation rate, cytotoxicity and myogenic differentiation potential were compared with those cultured in media supplemented with FBS. The results showed similar proliferation rates among human myoblasts cultured in PRP, PRP-HA or FBS supplemented media, with no cytotoxic effects. Human myoblasts cultured in PRP or PRP-HA showed reduced fusion ability upon differentiation. Nevertheless, we also observed that human MuRCs generated from PRP or PRP-HA myogenic cultures, exhibited increased Pax7 expression and delayed re-entry into the cell cycle upon reactivation, indicating a deeper quiescent state of human MuRCs. These results suggest that allogeneic human PRP effectively replaces FBS for the ex vivo expansion and differentiation of human myoblasts and favors the in vitro generation of Pax7High human MuRCs, with important implications for the advancement of stem cell-based muscle repair strategies.
© 2024. The Author(s).
-
Homo sapiens (Human)
In Frontiers in Immunology on 4 September 2024 by Siegel, M., Padamsey, A., et al.
Immunogenicity refers to the ability of a substance, such as a therapeutic drug, to elicit an immune response. While beneficial in vaccine development, undesirable immunogenicity can compromise the safety and efficacy of therapeutic proteins by inducing anti-drug antibodies (ADAs). These ADAs can reduce drug bioavailability and alter pharmacokinetics, necessitating comprehensive immunogenicity risk assessments starting at early stages of drug development. Given the complexity of immunogenicity, an integrated approach is essential, as no single assay can universally recapitulate the immune response leading to the formation of anti-drug antibodies.
To better understand the Dendritic Cell (DC) contribution to immunogenicity, we developed two flow cytometry-based assays: the DC internalization assay and the DC activation assay. Monocyte-derived dendritic cells (moDCs) were generated from peripheral blood mononuclear cells (PBMCs) and differentiated over a five-day period. The internalization assay measured the accumulation rate of therapeutic antibodies within moDCs, while the activation assay assessed the expression of DC activation markers such as CD40, CD80, CD86, CD83, and DC-SIGN (CD209). To characterize these two assays further, we used a set of marketed therapeutic antibodies.
The study highlights that moDCs differentiated for 5 days from freshly isolated monocytes were more prone to respond to external stimuli. The internalization assay has been shown to be highly sensitive to the molecule tested, allowing the use of only 4 donors to detect small but significant differences. We also demonstrated that therapeutic antibodies were efficiently taken up by moDCs, with a strong correlation with their peptide presentation on MHC-II. On the other hand, by monitoring DC activation through a limited set of activation markers including CD40, CD83, and DC-SIGN, the DC activation assay has the potential to compare a series of compounds. These two assays provide a more comprehensive understanding of DC function in the context of immunogenicity, highlighting the importance of both internalization and activation processes in ADA development.
The DC internalization and activation assays described here address key gaps in existing immunogenicity assessment methods by providing specific and reliable measures of DC function. The assays enhance our ability to pre-clinically evaluate the immunogenic potential of biotherapeutics, thereby improving their safety and efficacy. Future work should focus on further validating these assays and integrating them into a holistic immunogenicity risk assessment framework.
Copyright © 2024 Siegel, Padamsey, Bolender, Hargreaves, Fraidling, Ducret, Hartman, Looney, Bertinetti-Lapatki, Rohr, Hickling, Kraft and Marban-Doran.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
In International Journal of Molecular Medicine on 1 September 2024 by Kim, H. Y., Seo, I. K., et al.
CD150, also termed signaling lymphocyte activation molecule family member 1, is a cell surface receptor expressed on T cells, B cells, dendritic cells (DCs) and some tumors. Stimulation of CD150 on immune cells induces cell proliferation and cytokine production. However, the function of CD150 in Epstein‑Barr virus (EBV)‑infected B cells is still not fully understood. In the present study, CD150 expression on B cells increased rapidly following EBV infection, and various CD150 antibodies, measles viral proteins and recombinant CD150 proteins induced the secretion of multiple cytokines in both CD150+ EBV‑transformed B cells and EBV+ lymphoma cells. Notably, the IL‑1α protein level showed the greatest increase among all cytokines measured. The culture supernatant containing these cytokines induced the rapid differentiation of monocytes to DCs after only 2 days in vitro, which was faster than the established DC maturation time. Furthermore, knockdown of CD150 expression led to a reduction in the secretion of multiple cytokines, and monocyte differentiation was partially inhibited by anti‑IL‑1α and anti‑granulocyte‑macrophage colony‑stimulating factor neutralizing antibodies. Collectively, the results of the present study suggest that CD150 activation triggers cytokine production in EBV‑transformed B cells, and that measles virus coinfection might affect immune responses through the production of various cytokines in EBV+ lymphoma cells.
-
Cardiovascular biology
-
Immunology and Microbiology
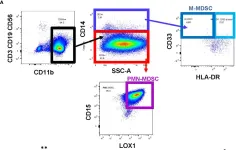
In Front Immunol on 30 October 2023 by Bazargan, S., Bunch, B., et al.
Fig.1.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 1