Mesenchymal stem cells (MSCs) are frequently used for therapeutic applications in both pre-clinical and clinical settings owing to their capacity for immune modulation and neuroprotective effects. However, transient fever is commonly observed as an adverse event following MSC injection in patients with Alzheimer's disease (AD). In this study, we investigated the potential impact of immunosuppressants such as dexamethasone and tacrolimus on altering the characteristics of human mesenchymal stem cells (hMSCs). Additionally, we examined whether these immunosuppressants affect the persistence of hMSCs or the immune response upon their administration into the brain parenchyma of AD mice. The exposure of hMSCs to high concentrations of dexamethasone and tacrolimus in vitro did not significantly alter the characteristics of hMSCs. The expression of genes related to innate immune responses, such as Irak1, Irf3, Nod1, and Ifnar1, was significantly downregulated by the additional administration of dexamethasone and tacrolimus to the brain parenchyma of AD mice. However, hMSC persistence in the AD mouse brain was not affected. The results of this study support the use of immunosuppressants to mitigate fever during stem cell therapy in patients with AD.
Product Citations: 166
In International Journal of Stem Cells on 30 May 2025 by Lee, N. K., Na, D. L., et al.
-
Stem Cells and Developmental Biology
In Nature Communications on 30 May 2025 by Wu, Z., Gao, S., et al.
Severe immune aplastic anemia is a fatal disease due to the destruction of marrow hematopoietic cells by cytotoxic lymphocytes, serving as a paradigm for marrow failure syndromes and autoimmune diseases. To better understand its pathophysiology, we apply advanced single cell methodologies, including mass cytometry, single-cell RNA, and TCR/BCR sequencing, to patient samples from a clinical trial of immunosuppression and growth factor stimulation. We observe opposing changes in the abundance of myeloid cells and T cells, with T cell clonal expansion dominated by effector memory cells. Therapy reduces and suppresses cytotoxic T cells, but new T cell clones emerge hindering robust hematopoietic recovery. Enhanced cell-cell interactions including between hematopoietic cells and immune cells, in particular evolving IFNG and IFNGR, are noted in patients and are suppressed post-therapy. Hematologic recovery occurs with increases in the progenitor rather than stem cells. Genetic predispositions linked to immune activation genes enhances cytotoxic T cell activity and crosstalk with target cells.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
-
Immunology and Microbiology
In Cellular and Molecular Biology (Noisy-le-Grand, France) on 26 May 2025 by Marure-Rojano, A. E., Cano-García, J. R., et al.
The pulmonary parenchyma is the primary site of metastasis for giant cell tumor (GCT) of bone, a benign yet aggressive musculoskeletal tumor. Current treatments, including surgery and antibody therapy, are only partially effective and often lead to significant side effects. This study aimed to evaluate the apoptotic activity of quercetin, a naturally occurring flavonoid with anticancer properties, on metastatic GCT lung cells (TIB-223). The immunophenotype of the TIB-223 cell line was characterized using flow cytometry, revealing positivity for CD166 and CD47 markers and negativity for CD34, CD73, CD117, CD45, and fibroblast markers. The IC50 of quercetin was determined at 91.1 µM through MTT assays, demonstrating its cytotoxic effect in a dose-dependent manner. Apoptosis was confirmed via flow cytometry and Western blotting, showing increased caspase-3 expression after 24 hours of treatment. These findings indicate that quercetin induces apoptosis in metastatic GCT cells and could serve as a basis for developing phytopharmaceutical therapies targeting this pathology.
-
Cancer Research
In Nature Protocols on 1 May 2025 by Li, Y. R., Zhou, K., et al.
The clinical potential of current chimeric antigen receptor-engineered T (CAR-T) cell therapy is hampered by its autologous nature that poses considerable challenges in manufacturing, costs and patient selection. This spurs demand for off-the-shelf therapies. Here we introduce an ex vivo feeder-free culture method to differentiate gene-engineered hematopoietic stem and progenitor (HSP) cells into allogeneic invariant natural killer T (AlloNKT) cells and their CAR-armed derivatives (AlloCAR-NKT cells). We include detailed information on lentivirus generation and titration, as well as the five stages of ex vivo culture required to generate AlloCAR-NKT cells, including HSP cell engineering, HSP cell expansion, NKT cell differentiation, NKT cell deep differentiation and NKT cell expansion. In addition, we describe procedures for evaluating the pharmacology, antitumor efficacy and mechanism of action of AlloCAR-NKT cells. It takes ~2 weeks to generate and titrate lentiviruses and ~6 weeks to generate mature AlloCAR-NKT cells. Competence with human stem cell and T cell culture, gene engineering and flow cytometry is required for optimal results.
© 2025. Springer Nature Limited.
-
Cancer Research
-
Immunology and Microbiology
In Nature Biotechnology on 1 March 2025 by Li, Y. R., Zhou, Y., et al.
Cancer immunotherapy with autologous chimeric antigen receptor (CAR) T cells faces challenges in manufacturing and patient selection that could be avoided by using 'off-the-shelf' products, such as allogeneic CAR natural killer T (AlloCAR-NKT) cells. Previously, we reported a system for differentiating human hematopoietic stem and progenitor cells into AlloCAR-NKT cells, but the use of three-dimensional culture and xenogeneic feeders precluded its clinical application. Here we describe a clinically guided method to differentiate and expand IL-15-enhanced AlloCAR-NKT cells with high yield and purity. We generated AlloCAR-NKT cells targeting seven cancers and, in a multiple myeloma model, demonstrated their antitumor efficacy, expansion and persistence. The cells also selectively depleted immunosuppressive cells in the tumor microenviroment and antagonized tumor immune evasion via triple targeting of CAR, TCR and NK receptors. They exhibited a stable hypoimmunogenic phenotype associated with epigenetic and signaling regulation and did not induce detectable graft versus host disease or cytokine release syndrome. These properties of AlloCAR-NKT cells support their potential for clinical translation.
© 2024. The Author(s).
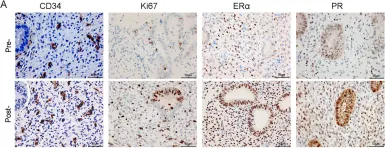
In Stem Cell Res Ther on 22 July 2021 by Zhang, Y., Shi, L., et al.
Fig.4.A

-
IHC
-
Collected and cropped from Stem Cell Res Ther by CiteAb, provided under a CC-BY license
Image 1 of 1