Adipose-derived stromal/stem cells (ASCs) are promising for the treatment of many diseases, including tissue injury or degeneration associated with serious disease and high morbidity. The extent of cell therapy effectiveness, however, may be limited by the lower survival of implanted cells in environments of tissue damage. Therefore, strategies to improve cell survival are important, such as by pretreating/preconditioning cells with a beneficial agent. We investigated the pretreatment of human ASCs (hASCs) with StemRegenin 1 (SR1), a purine derivative used in clinical protocols for in vitro hematopoietic stem/progenitor cell expansion. We pretreated hASCs with SR1 and analyzed the resulting cells (SR1-hASCs) as compared to non-treated cells (NT-hASCs). We noted that treatment with SR1 significantly increased the proliferation and migration of hASCs, as well as their secretion of paracrine factors of interest, and did not affect their cell differentiation capacity. Furthermore, when these SR1-hASCs were subsequently exposed to antimycin A, a mitochondrial respiratory chain inhibitor, they showed significantly higher antioxidative, anti-apoptotic, and pro-survival abilities as compared to NT-hASCs. Since oxidative stress and other harsh environments result from tissue damage, our results support that the preconditioning of hASCs with SR1 may enhance their protective, reparative, and regenerative, and thus therapeutic, efficacy.
© 2025 The Authors.
Product Citations: 205
In Molecular Therapy. Methods Clinical Development on 11 December 2025 by Zhao, J., Yu, B., et al.
-
Stem Cells and Developmental Biology
Functional and molecular analyses reveal impaired HSPCs in Multiple Myeloma patients post-induction.
In Stem Cells Translational Medicine on 14 November 2025 by Baumhardt, T. M., Amoah, A., et al.
High-dose chemotherapy and consecutive autologous stem cell transplantation (ASCT) remain the backbone of treatment for transplant-eligible patients of Multiple Myeloma (MM). However, patients are still at high risk of relapse or treatment-related complications. Hence, by understanding the function of hematopoietic stem and progenitor cells (HSPCs) from MM patients in more detail, transplant outcomes in MM patients might be further improved. We combine in our study functional analyses of the potential of HSPCs from newly diagnosed (NDMM) and chemotherapy treated MM patients in a xenotransplant model system with in depth single cells sequencing analysis to provide novel data that might inform clinical routine to improve the outcome of ASCT in MM. Our data demonstrate that (i) HSPCs from treated MM patients are indeed significantly impaired in their overall reconstitution potential and provide a reduced level of B-cells in comparison to HSPCs from age-matched healthy donors and NDMM patients. (ii) We further demonstrate that CD34+ HSPCs acquire a high-risk MM expression profile signature upon induction treatment, which likely adds to the risk of relapse. This high-risk MM expression profile signature relies within CD34+ HSPCs primarily in granulocyte/macrophage progenitors (GMPs), megakaryocyte Erythroid Progenitors (MEPs) and monocytes, while hematopoietic stem cells (HSCs) stay unaffected by transcriptional changes. These data suggest that the elimination of myeloid progenitors and more mature monocytes (likely by purification for HSCs) in HSPCs harvests from treated MM patients for subsequent ASCT might improve transplant outcomes by avoiding re-infusion of cells with a dysregulated and disease-linked transcriptional program.
© The Author(s) 2025. Published by Oxford University Press.
-
Stem Cells and Developmental Biology
Aberrant splicing of MBD1 reshapes the epigenome to drive convergent myeloerythroid defects in MDS
Preprint on BioRxiv : the Preprint Server for Biology on 19 October 2025 by Chen, H. T. (., Joshi, P., et al.
Myelodysplastic neoplasms (MDS) feature hematopoietic deficits driven in part by transcript splicing abnormalities. Thus far, such disease-driving transcripts have been identified in association with specific splicing factor mutations. However, it remains unclear whether there also exists a set of disease-wide conserved pathological transcripts, which drive MDS independently of mutational status. Here, we characterize an MDS-associated long isoform of MBD1 (MBD1-L) as the first described member of this class of transcripts. Overexpression of MBD1-L in healthy human HSPCs recapitulates archetypal defects of MDS including deficits in erythroid differentiation and reconstitution capacity. These defects arise from an isoform-specific switching of MBD1’s binding behavior, refocusing its heterochromatin-promoting activity from methylated to unmethylated CpGs and enacting broad downregulation of CpG-rich promoters as well as secondary epigenetic effects mediated by its downstream target BCOR . Remarkably, we also find that directly reversing abnormal MBD1 splicing in primary human MDS using nanoparticle-encapsulated ASOs enhances erythroid differentiation. Key points Global mis-splicing of MBD1 represents a novel gain-of-function epigenetic axis driving erythropoietic and proliferative defects in MDS. ASO based depletion of pathogenic MBD1 transcripts restores erythroid differentiation, advancing RNA-based therapies for MDS.
Protocol for generating liver organoids containing Kupffer cells using human iPSCs.
In STAR Protocols on 10 October 2025 by Li, Y., Nie, Y., et al.
Integration of resident immune cells into in vitro organoid models is important for accurately recapitulating native tissue physiology. Here, we present a protocol for integrating liver-resident macrophages, Kupffer cells, into liver organoid models derived from human induced pluripotent stem cells (iPSCs). We describe procedures for generating Kupffer cell progenitors and hepatic endoderm from iPSCs, followed by detailed steps for establishing liver organoids containing Kupffer cells (KuLOs). This protocol provides a platform for investigating the roles of Kupffer cells in liver development and diseases. For complete details on the use and execution of this protocol, please refer to Li et al.1.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
In Scientific Reports on 12 August 2025 by Sutjarit, N., Ruknarong, L., et al.
Umbilical cord blood (UCB) units are an alternative source of human hematopoietic stem cells (HSCs) for allogeneic stem cell transplants. A large quantity of HSCs is needed but the low number of accessible cells from UCB has been a significant limitation. Improving the ex vivo growth of HSCs while preserving their functioning is required. Here, we report that andrographolide (AP) enhanced the expansion of human UCB-derived HSCs (HSPCs) and pro-moted primitive HSCs (CD34+CD38-CD90+). AP also improved HSC functionality, evidenced by increased growth of colony-forming units and multilineage differentiation. AP upregulated genes involved in the Wnt/β-catenin and Notch signaling pathways. AP also modulated signaling pathways involved in HSC self-renewal, proliferation, survival, and differentiation, demonstrated by Nanostring analysis. The results of this study suggest that andrographolide enhances ex vivo UCB-HSC expansion while maintaining functionality and has potential for treatment of hematological diseases.
© 2025. The Author(s).
-
Cardiovascular biology
-
Stem Cells and Developmental Biology
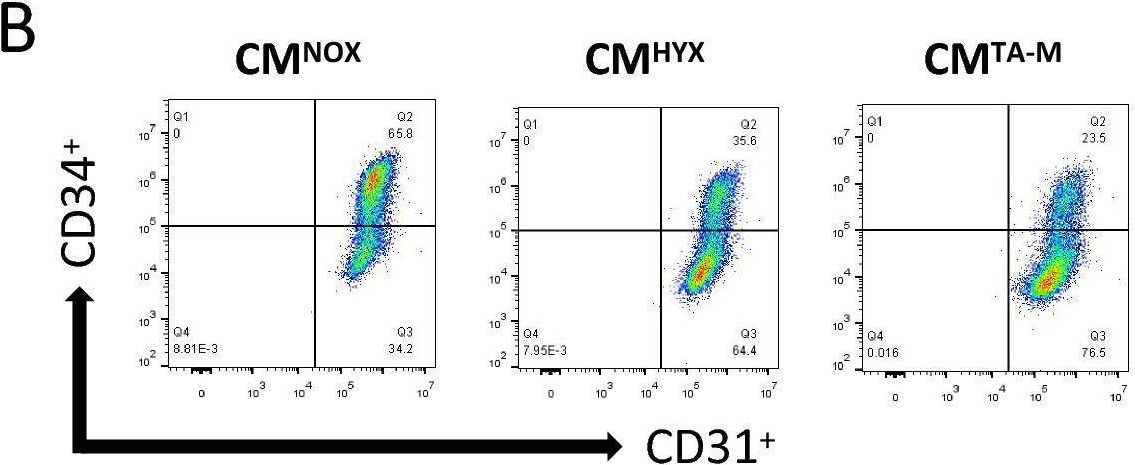
In Stem Cell Res Ther on 11 September 2024 by Wang, X., Yao, F., et al.
Fig.2.B
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Stem Cell Research & Therapy by CiteAb, provided under a CC-BY license
Image 1 of 7
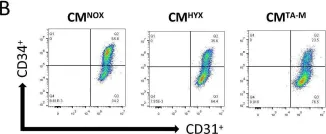
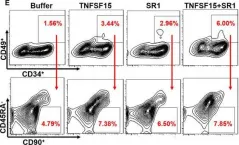
In J Cell Mol Med on 1 October 2020 by Ding, Y., Gao, S., et al.
Fig.1.D
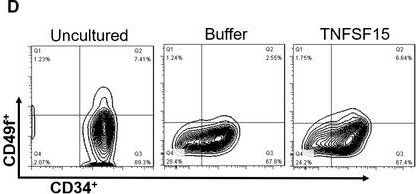
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Journal of Cellular and Molecular Medicine by CiteAb, provided under a CC-BY license
Image 1 of 7
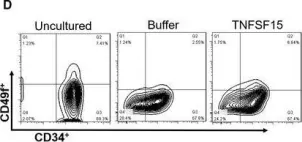
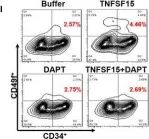
In J Cell Mol Med on 1 October 2020 by Ding, Y., Gao, S., et al.
Fig.1.H
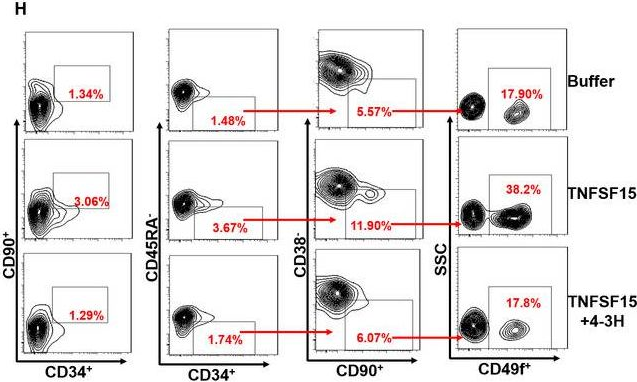
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Journal of Cellular and Molecular Medicine by CiteAb, provided under a CC-BY license
Image 1 of 7
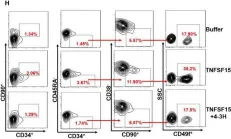
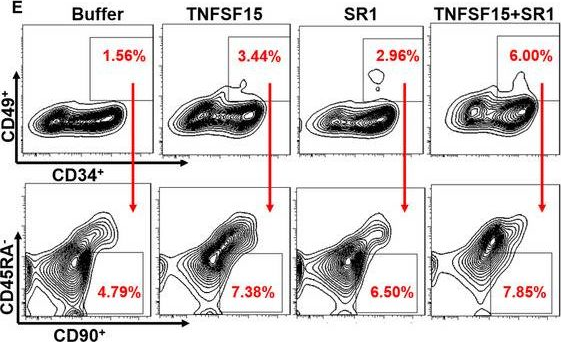
In J Cell Mol Med on 1 October 2020 by Ding, Y., Gao, S., et al.
Fig.3.A
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Journal of Cellular and Molecular Medicine by CiteAb, provided under a CC-BY license
Image 1 of 7
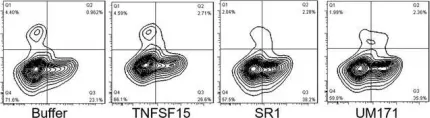
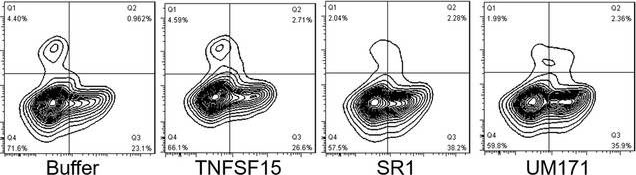
In J Cell Mol Med on 1 October 2020 by Ding, Y., Gao, S., et al.
Fig.3.E
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Journal of Cellular and Molecular Medicine by CiteAb, provided under a CC-BY license
Image 1 of 7
In J Cell Mol Med on 1 October 2020 by Ding, Y., Gao, S., et al.
Fig.5.L
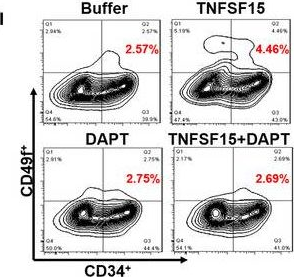
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Journal of Cellular and Molecular Medicine by CiteAb, provided under a CC-BY license
Image 1 of 7
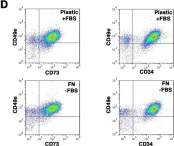
In Sci Rep on 14 March 2017 by Di Maggio, N., Martella, E., et al.
Fig.5.D
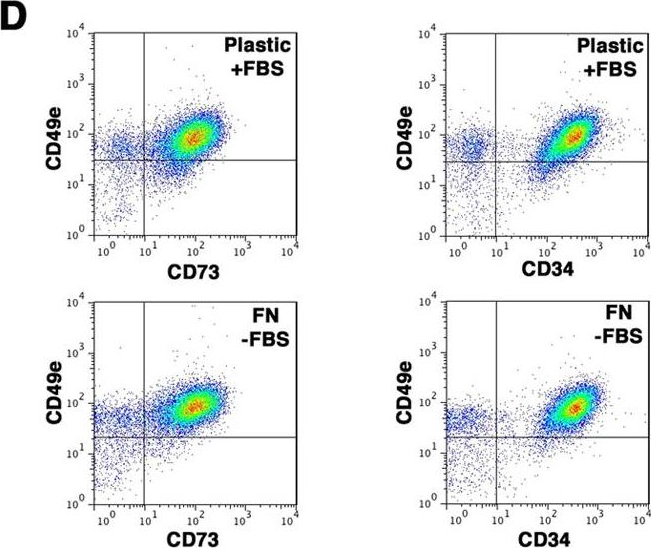
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Scientific Reports by CiteAb, provided under a CC-BY license
Image 1 of 7