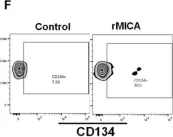
Mucosal-associated invariant T cells (MAIT cells) are a subset of T cells with innate, effector-like properties that play an essential role in the immune response to microbial infections. In humans, MAIT cells are detectable in the blood, liver, and lungs, but little is known about the frequency of these cells in the bone marrow. Also, the pathogenic role, if any, of MAIT cells in the development of aplastic anemia, a disease with an exquisite origin in the bone marrow, is currently unknown. We investigated the frequency and clinical relevance of bone marrow MAIT cells in a cohort of 14 patients (60.6 ± 23 and 57% women) with aplastic anemia. MAIT cells in the bone marrow samples obtained at diagnosis were evaluated by flow cytometry, and their association with various blood cell parameters and the patients' clinical features was analyzed. MAIT cells were detectable in the bone marrow of all patients, with considerable variations among them. Bone marrow MAIT cells expressing the activator receptor natural killer group 2D - NKG2D (NKG2D+ MAIT cells) were significantly more abundant in the specimens of the aplastic anemia patients than in patients with bone marrow failure distinct from aplastic anemia. In addition, the NKG2D+ MAIT cells positively correlated with whole blood cell counts (WBC), platelet counts, and neutrophil counts, as well as with various inflammatory markers, including neutrophil-to-lymphocyte rate (NLR), platelet-to-lymphocyte rate (PLR), and systemic inflammatory index (SII). In functional studies, bone marrow CD34+ hematopoietic cells exposed to phytohemagglutinin or bacterial-derived lipopolysaccharide and acetyl-6-formylpterin upregulated MR1 (major histocompatibility complex, class I-related, known to interact with MAIT cells) and MICA/B (MHC class I chain-related gene A, a ligand of NKG2D) proteins on their cell surface, suggesting that under stress conditions, CD34+ hematopoietic cells are more likely to interact with NKG2D+ MAIT cells. In addition, NKG2D+ MAIT cells upregulated perforin and granzyme B in response to their interaction with recombinant MICA protein in vitro. This study reports for the first time the frequency of MAIT cells in the bone marrow of patients with aplastic anemia and assesses the potential implications of these cells in the pathogenesis or progression of aplastic anemia.
Product Citations: 12
MAIT Cells in the Bone Marrow of Patients with Aplastic Anemia.
In International Journal of Molecular Sciences on 21 September 2024 by Lam, V. Q., Espinoza, J. L., et al.
-
FC/FACS
-
Homo sapiens (Human)
In International Immunopharmacology on 1 November 2022 by Ma, Y., Liu, F., et al.
Coronavirus disease 2019 (COVID-19) continues to be a major global public health challenge, with the emergence of variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Current vaccines or monoclonal antibodies may not well be protect against infection with new SARS-CoV-2 variants. Unlike antibody-based treatment, T cell-based therapies such as TCR-T cells can target epitopes that are highly conserved across different SARS-CoV-2 variants. Reportedly, T cell-based immunity alone can restrict SARS-CoV-2 replication.
In this study, we identified two TCRs targeting the RNA-dependent RNA polymerase (RdRp) protein in CD8 + T cells. Functional evaluation by transducing these TCRs into CD8 + or CD4 + T cells confirmed their specificity.
Combinations of inflammatory and anti-inflammatory cytokines secreted by CD8 + and CD4 + T cells can help control COVID-19 in patients. Moreover, the targeted epitope is highly conserved in all emerged SARS-CoV-2 variants, including the Omicron. It is also conserved in the seven coronaviruses that infect humans and more broadly in the subfamily Coronavirinae.
The pan-genera coverage of mutant epitopes from the Coronavirinae subfamily by the two TCRs highlights the unique strengths of TCR-T cell therapies in controlling the ongoing pandemic and in preparing for the next coronavirus outbreak.
Copyright © 2022 Elsevier B.V. All rights reserved.
-
FC/FACS
-
COVID-19
-
Immunology and Microbiology
In Cancer Immunology Research on 3 August 2022 by Kim, S. P., Vale, N. R., et al.
Adoptive cellular therapy (ACT) targeting neoantigens can achieve durable clinical responses in patients with cancer. Most neoantigens arise from patient-specific mutations, requiring highly individualized treatments. To broaden the applicability of ACT targeting neoantigens, we focused on TP53 mutations commonly shared across different cancer types. We performed whole-exome sequencing on 163 patients with metastatic solid cancers, identified 78 who had TP53 missense mutations, and through immunologic screening, identified 21 unique T-cell reactivities. Here, we report a library of 39 T-cell receptors (TCR) targeting TP53 mutations shared among 7.3% of patients with solid tumors. These TCRs recognized tumor cells in a TP53 mutation- and human leucocyte antigen (HLA)-specific manner in vitro and in vivo. Twelve patients with chemorefractory epithelial cancers were treated with ex vivo-expanded autologous tumor-infiltrating lymphocytes (TIL) that were naturally reactive against TP53 mutations. However, limited clinical responses (2 partial responses among 12 patients) were seen. These infusions contained low frequencies of mutant p53-reactive TILs that had exhausted phenotypes and showed poor persistence. We also treated one patient who had chemorefractory breast cancer with ACT comprising autologous peripheral blood lymphocytes transduced with an allogeneic HLA-A*02-restricted TCR specific for p53R175H. The infused cells exhibited an improved immunophenotype and prolonged persistence compared with TIL ACT and the patient experienced an objective tumor regression (-55%) that lasted 6 months. Collectively, these proof-of-concept data suggest that the library of TCRs targeting shared p53 neoantigens should be further evaluated for the treatment of patients with advanced human cancers. See related Spotlight by Klebanoff, p. 919.
©2022 The Authors; Published by the American Association for Cancer Research.
-
FC/FACS
-
Cancer Research
-
Immunology and Microbiology
In Nature Communications on 16 February 2021 by Duhen, R., Ballesteros-Merino, C., et al.
Despite the success of checkpoint blockade in some cancer patients, there is an unmet need to improve outcomes. Targeting alternative pathways, such as costimulatory molecules (e.g. OX40, GITR, and 4-1BB), can enhance T cell immunity in tumor-bearing hosts. Here we describe the results from a phase Ib clinical trial (NCT02274155) in which 17 patients with locally advanced head and neck squamous cell carcinoma (HNSCC) received a murine anti-human OX40 agonist antibody (MEDI6469) prior to definitive surgical resection. The primary endpoint was to determine safety and feasibility of the anti-OX40 neoadjuvant treatment. The secondary objective was to assess the effect of anti-OX40 on lymphocyte subsets in the tumor and blood. Neoadjuvant anti-OX40 was well tolerated and did not delay surgery, thus meeting the primary endpoint. Peripheral blood phenotyping data show increases in CD4+ and CD8+ T cell proliferation two weeks after anti-OX40 administration. Comparison of tumor biopsies before and after treatment reveals an increase of activated, conventional CD4+ tumor-infiltrating lymphocytes (TIL) in most patients and higher clonality by TCRβ sequencing. Analyses of CD8+ TIL show increases in tumor-antigen reactive, proliferating CD103+ CD39+ cells in 25% of patients with evaluable tumor tissue (N = 4/16), all of whom remain disease-free. These data provide evidence that anti-OX40 prior to surgery is safe and can increase activation and proliferation of CD4+ and CD8+ T cells in blood and tumor. Our work suggests that increases in the tumor-reactive CD103+ CD39+ CD8+ TIL could serve as a potential biomarker of anti-OX40 clinical activity.
-
FC/FACS
-
Cancer Research
-
Immunology and Microbiology
In Cancer Science on 1 June 2020 by Suzuki, S., Ogawa, T., et al.
Immune-checkpoint inhibitors improve the survival of head and neck squamous cell carcinoma (HNSCC) patients. Although recent studies have demonstrated that the tumor immune microenvironment (TIME) has critical roles in immunotherapy, the precise mechanisms involved are unclear. Therefore, further investigations of TIME are required for the improvement of immunotherapy. The frequency of effector regulatory T-cells (eTregs) and the expression of immune-checkpoint molecules (ICM) on eTregs and conventional T-cells (Tconvs) both in peripheral blood lymphocytes (PBL) and tumor-infiltrating lymphocytes (TIL) from HNSCC patients were analyzed by flow cytometry and their distributions were evaluated by multi-color immunofluorescence microscopy. High frequency eTreg infiltration into HNSCC tissues was observed and high expressions of CD25, FOXP3, stimulatory-ICM (4-1BB, ICOS, OX40 and GITR) and inhibitory-ICM (programmed cell death-1 [PD-1] and cytotoxic T-lymphocyte-associated protein-4 [CTLA-4]) were found on invasive eTregs. In contrast, the expression of stimulatory-ICM on Tconvs was low and the expression of inhibitory-ICM was high. In addition, ICM-ligands (programmed cell death-1 [PD-L1], galectin-9 and CEACAM-1) were frequently expressed on cancer cells. PD-L1 and galectin-9 were also expressed on macrophages. PD-1+ T-cells interacted with PD-L1+ cancer cells or PD-L1+ macrophages. This suggested that in TIL, eTregs are highly activated, but Tconvs are exhausted or inactivated by eTregs and immune-checkpoint systems, and ICM and eTregs are strongly involved in the creation of an immunosuppressive environment in HNSCC tissues. These suggested eTreg targeting drugs are expected to be a combination partner with immune-checkpoint inhibitors that will improve immunotherapy of HNSCC.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
-
Cancer Research
-
Immunology and Microbiology
In Int J Mol Sci on 21 September 2024 by Lam, V. Q., Espinoza, J. L., et al.
Fig.3.F

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 1