The chronic inflammatory component of asthma is propagated by granulocytes, including neutrophils and eosinophils, in the peripheral circulation and airway. Previous studies have suggested that these cells have an altered expression of adhesion-related molecules and a propensity for the release of granule contents that may contribute to tissue damage and enhance inflammatory complications in patients with status asthmaticus. The goal of this prospective cohort study at a tertiary care pediatric hospital with a large population of asthma patients was to assess the role of granulocyte-based inflammation in the development of asthma exacerbation. Subjects were enrolled from two patient populations: those with mild-to-moderate asthma exacerbations seen in the emergency department and those with severe asthma admitted to the intensive care unit (PICU). Clinical data were collected, and blood was drawn. Granulocytes were immediately purified, and the phenotype was assessed, including the expression of cell surface markers, elastase release, and cytokine production. Severe asthmatics admitted to the PICU displayed a significantly higher total neutrophil count when compared with healthy donors. Moreover, little to no eosinophils were found in granulocyte preparations from severe asthmatics. Circulating neutrophils from severe asthmatics admitted to the PICU displayed significantly increased elastase release ex vivo when compared with the PMN from healthy donors. These data suggest that the neutrophil-based activation and release of inflammatory products displayed by severe asthmatics may contribute to the propagation of asthma exacerbations.
Product Citations: 9
In Cells on 18 March 2024 by Henley, K., Tresselt, E., et al.
-
Homo sapiens (Human)
-
Cell Biology
In Frontiers in Pharmacology on 1 May 2023 by Wagner, A., Pehar, M., et al.
Recent interest in mushrooms and their components as potential therapies for mental health, along with recent government and health authority approvals, has necessitated a more comprehensive understanding of their effects on the cellular microenvironment of the brain. Amanita muscaria has been ingested as a treatment for a variety of ailments for centuries, most notably those affecting the central nervous system and conditions associated with neuroinflammation. However, the effects of these extracts on neuroinflammatory cells, such as microglia, are unknown. The effect of commercially-sourced A. muscaria extract (AME-1) on human microglial cell line (HMC3) expression of surface receptors such as CD86, CXCR4, CD45, CD125 and TLR4 was determined by flow cytometry. AME-1 upregulated expression of all of these receptors. The effect of AME-1 on HMC3 production of IL-8 and IL-6 was determined and compared to tumor necrosis factor (TNF), polyinosinic-polycytidylic acid [poly(I:C)], substance P and lipopolysaccharide (LPS), all known activators of HMC-3 and primary microglia. HMC3 produced both IL-8 and IL-6 when activated with LPS, TNF and poly(I:C) but not when they were activated with substance P. Although AME-1 at higher concentrations increased IL-8 production of HMC3 on its own, AME-1 notably potentiated HMC3 production of IL-8 in response to poly(I:C). AME-1 altered expression of toll-like receptor 3 (TLR3) mRNA but not surface protein by HMC3. AME-1 also did not significantly alter expression of retinoic acid-inducible gene I (RIG-I) or melanoma differentiation-associated protein 5 (MDA5), both cytosolic sensors of dsRNA. Metabolomics analysis showed that AME-1 contained several metabolites, including the autophagy inducer, trehalose. Like AME-1, trehalose also potentiated HMC3 poly(I:C) mediated production of IL-8. This study suggests that A. muscaria extracts can modify HMC3 inflammatory responses, possibly due to their trehalose content.
Copyright © 2023 Wagner, Pehar, Yan and Kulka.
-
FC/FACS
-
Neuroscience
-
Pharmacology
In Journal of Immunological Methods on 1 August 2022 by Pioch, J. & Blomgran, R.
Reactive oxygen species (ROS) and the ability of immune cells to mount an oxidative burst represent an important defense during microbial invasion, but is also recognized for playing a significant role in the progression of inflammatory disorders and disease. Although neutrophils produce the strongest ROS-response, other leukocytes and their cell subsets could play a significant role. Isolation of specific cells for determining their ROS-response can affect their functionality and is laborious or hard to replicate in different settings. We have therefore established a whole blood assay, that only requires 100 μL heparinized blood and utilizes the dihydrorhodamine (DHR) 123 ROS-probe combined with cell surface antibody staining for the specific detection of ROS in several subsets of cells simultaneously using flow cytometry. Although the flow markers chosen are interchangeable with other direct conjugated and cell specific antibodies depending on the research question, we focused on neutrophils (SSChighCD16brightHLA-DRneg/low), eosinophils (SSChighCD16lowHLA-DRlow/negCD193positiveCD125positive) and monocyte subsets (SSCintermediateHLA-DRhighCD14low-positiveCD16negative-positive). As a RBC-lysis reagent we compared BD FACS Lysis Solution to the in-house prepared ammonium-chloride‑potassium based ACK Lysis Buffer, that does not fix or permeabilize the immune cells. We find that ACK-lysis of stimulated and stained samples results in superior surface staining, decreased loss of cell subsets, and enhanced resolution of the DHR-signal. Compared to the other cells analyzed in healthy blood donors, neutrophils responded with the highest ROS-response to all tested stimuli (fMLP (low stimuli), E. coli, and PMA (high stimuli)), where eosinophils and the three monocyte subsets also showed an extensive ROS-response when stimulated with E. coli or PMA. Our assay provides the possibility for researchers to examine the ROS-response of specific cell subsets in specific patient groups ex vivo and could also allow the analysis of pharmacological intervention studies targeting ROS, which ultimately can advance the field of immunological research.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
-
Cardiovascular biology
-
Immunology and Microbiology
Eosinophil-derived neurotoxin: A biologically and analytically attractive asthma biomarker.
In PLoS ONE on 11 February 2021 by Rutten, B., Young, S., et al.
There is a growing body of evidence for the utility of eosinophil-derived neurotoxin (EDN) as a biomarker in asthma, including association with eosinophilic airway inflammation, assessment of disease severity and potential for predicting pathogenic risks, including exacerbations. However, to interpret any biomarker data with confidence, it is first important to understand the preanalytical factors and biological variation that may affect its reliable measurement and results interpretation. In this study we defined the healthy serum EDN reference range for men and women as 1.98 to 26.10 ng/mL, with no significant gender differences. Smoking did not impact the mean EDN levels and no circadian rhythm was identified for EDN, unlike blood eosinophils (EOS) where levels peaked at 00:00h. EDN expression in different cell types was investigated and shown to occur primarily in eosinophils, indicating they are likely to be the main cellular repository for EDN. We also confirm that the quantification of serum EDN is not influenced by the type of storage tube used, and it is stable at ambient temperature or when refrigerated for at least 7 days and for up to one year when frozen at -20°C or -80°C. In summary, EDN is a stable biomarker that may prove useful in precision medicine approaches by enabling the identification of a subpopulation of asthma patients with activated eosinophils and a more severe form of the disease.
-
Homo sapiens (Human)
In Frontiers in Immunology on 26 January 2021 by Kim, J. E., Lee, D. H., et al.
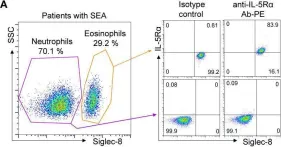
Patients with severe eosinophilic asthma (SEA; characterized by persistent eosinophilia in blood and airway tissues) experience frequent asthma exacerbations with poor clinical outcomes. Interleukin 5 (IL-5) and IL-5 receptor alpha subunit (IL-5α) play key roles in eosinophilia maintenance, and relevant therapeutic strategies include the development of antibodies (Abs) against IL-5 or IL-5α to control eosinophilia. Benralizumab, an anti-IL-5α Ab that depletes eosinophils mainly via Ab-dependent cell-mediated cytotoxicity and through blockage of IL-5 function on eosinophils, has been clinically approved for patients with SEA. Here, we report engineering of a new humanized anti-IL-5Rα Ab with potent biological activity. We first raised murine Abs against human IL-5Rα, humanized a leading murine Ab, and then further engineered the humanized Abs to enhance their affinity for IL-5Rα using the yeast surface display technology. The finally engineered version of the Ab, 5R65.7, with affinity (KD ≈ 4.64 nM) stronger than that of a clinically relevant benralizumab analogue (KD ≈ 26.8 nM) showed improved neutralizing activity toward IL-5-dependent cell proliferation in a reporter cell system. Domain level Ab epitope mapping revealed that 5R65.7 recognizes membrane-proximal domain 3 of IL-5Rα, distinct from domain I epitope of the benralizumab analogue. In ex vivo assays with peripheral eosinophils from patients with SEA and healthy donors, 5R65.7 manifested more potent biological activities than the benralizumab analogue did, including inhibition of IL-5-dependent proliferation of eosinophils and induction of eosinophil apoptosis through autologous natural-killer-cell-mediated Ab-dependent cell-mediated cytotoxicity. Our study provides a potent anti-IL-5Rα Ab, 5R65.7, which is worthy of further testing in preclinical and clinical trials against SEA as a potential alternative to the current therapeutic arsenal.
Copyright © 2021 Kim, Lee, Jung, Kim, Choi, Park and Kim.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Front Immunol on 26 January 2021 by Kim, J. E., Lee, D. H., et al.
Fig.4.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 1