The abnormally thick glycocalyx of cancer cells can provide a physical barrier to immune cell recognition and effective immunotherapy. Here, we demonstrate an optical method based on Scanning Angle Interference Microscopy (SAIM) for the screening of therapeutic agents that can disrupt the glycocalyx layer as a strategy to improve anti-cancer immune responses. We developed a new membrane labeling strategy utilizing leucine zipper pairs to fluorescently mark the glycocalyx layer boundary for precise and robust measurement of glycocalyx thickness with SAIM. Using this platform, we evaluated the effects of glycosylation inhibitors and targeted enzymatic degraders of the glycocalyx, with particular focus on strategies for cholangiocarcinoma (CCA), a highly lethal malignancy with limited therapeutic options. We found that CCA had the highest mean expression of the cancer-associated mucin, MUC1, across all cancers represented in the cancer cell line encyclopedia. Pharmacological inhibitors of mucin-type O-glycosylation and mucin-specific proteases, such as StcE, could dramatically reduce the glycocalyx layer in the YSCCC model of intrahepatic CCA. Motivated by these findings, we engineered Natural Killer (NK) cells tethered with StcE to enhance NK cell-mediated cytotoxicity against CCA. In a CCA xenograft model, these engineered NK cells demonstrated superior anti-tumor efficacy compared to wild-type NK cells, with no observable adverse effects. Our findings not only provide a reliable imaging-based screening platform for evaluating glycocalyx-targeting pharmacological interventions but also offer mechanistic insights into how CCA may avoid immune elimination through fortification of the glycocalyx layer with mucins. Additionally, this work presents a novel therapeutic strategy for mucin-overexpressing cancers, potentially improving immunotherapy efficacy across various cancer types.
Product Citations: 16
Preprint on BioRxiv : the Preprint Server for Biology on 6 December 2024 by Park, S., Paek, J. H., et al.
-
FC/FACS
-
Cancer Research
-
Immunology and Microbiology
In Proceedings of the National Academy of Sciences of the United States of America on 14 May 2024 by Goyette, M. A., Stevens, L. E., et al.
Brain metastatic breast cancer is particularly lethal largely due to therapeutic resistance. Almost half of the patients with metastatic HER2-positive breast cancer develop brain metastases, representing a major clinical challenge. We previously described that cancer-associated fibroblasts are an important source of resistance in primary tumors. Here, we report that breast cancer brain metastasis stromal cell interactions in 3D cocultures induce therapeutic resistance to HER2-targeting agents, particularly to the small molecule inhibitor of HER2/EGFR neratinib. We investigated the underlying mechanisms using a synthetic Notch reporter system enabling the sorting of cancer cells that directly interact with stromal cells. We identified mucins and bulky glycoprotein synthesis as top-up-regulated genes and pathways by comparing the gene expression and chromatin profiles of stroma-contact and no-contact cancer cells before and after neratinib treatment. Glycoprotein gene signatures were also enriched in human brain metastases compared to primary tumors. We confirmed increased glycocalyx surrounding cocultures by immunofluorescence and showed that mucinase treatment increased sensitivity to neratinib by enabling a more efficient inhibition of EGFR/HER2 signaling in cancer cells. Overexpression of truncated MUC1 lacking the intracellular domain as a model of increased glycocalyx-induced resistance to neratinib both in cell culture and in experimental brain metastases in immunodeficient mice. Our results highlight the importance of glycoproteins as a resistance mechanism to HER2-targeting therapies in breast cancer brain metastases.
-
Cancer Research
Immunoengineering can overcome the glycocalyx armour of cancer cells.
In Nature Materials on 1 March 2024 by Park, S., Colville, M. J., et al.
Cancer cell glycocalyx is a major line of defence against immune surveillance. However, how specific physical properties of the glycocalyx are regulated on a molecular level, contribute to immune evasion and may be overcome through immunoengineering must be resolved. Here we report how cancer-associated mucins and their glycosylation contribute to the nanoscale material thickness of the glycocalyx and consequently modulate the functional interactions with cytotoxic immune cells. Natural-killer-cell-mediated cytotoxicity is inversely correlated with the glycocalyx thickness of the target cells. Changes in glycocalyx thickness of approximately 10 nm can alter the susceptibility to immune cell attack. Enhanced stimulation of natural killer and T cells through equipment with chimeric antigen receptors can improve the cytotoxicity against mucin-bearing target cells. Alternatively, cytotoxicity can be enhanced through engineering effector cells to display glycocalyx-editing enzymes, including mucinases and sialidases. Together, our results motivate the development of immunoengineering strategies that overcome the glycocalyx armour of cancer cells.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
-
Cancer Research
Bulky glycocalyx drives cancer invasiveness by modulating substrate-specific adhesion
Preprint on BioRxiv : the Preprint Server for Biology on 6 August 2023 by Barai, A., Piplani, N., et al.
Majority of the eukaryotic cell surface is decorated with a layer of membrane attached polysaccharides and glycoproteins collectively referred to as the glycocalyx. While formation of a bulky glycocalyx has been associated with cancer progression, the mechanisms by which the glycocalyx regulates cancer invasiveness is incompletely understood. We address this question by first documenting sub-type specific expression of the major glycocalyx glycoprotein Mucin-1 (MUC1) in breast cancer patient samples and breast cancer cell lines. Strikingly, glycocalyx disruption led to inhibition of 2D motility, loss of 3D invasion and reduction of clonal scattering of breast cancer cells at the population level. Tracking of 2D cell motility and 3D invasiveness of MUC1-based sorted sub-populations revealed fastest motility and invasiveness in intermediate MUC1-expressing cells, with glycocalyx disruption abolishing these effects. While differential sensitivity in 2D motility is attributed to a non-monotonic dependence of focal adhesion size on MUC1 levels, higher MUC1 levels enhance 3D invasiveness via increased traction generation. In contrast to inducing cell rounding on collagen-coated substrates, high MUC1 level promotes cell adhesion and confers resistance to shear flow on substrates coated with the endothelial surface protein E-selectin. Collectively, our findings illustrate how MUC1 drives cancer invasiveness by differentially regulating cell-substrate adhesion in a substrate-dependent manner.
-
Cancer Research
Mucins form a nanoscale material barrier against immune cell attack
Preprint on Research Square on 3 August 2022 by Park, S., Colville, M., et al.
The cancer cell glycocalyx serves as a major line of defense against immune surveillance. However, how specific physical properties of the glycocalyx are regulated and contribute to immune evasion is not well understood. Here, we uncover how the surface density, glycosylation, and crosslinking of cancer-associated mucins contribute to the nanoscale material thickness of the glycocalyx, and further analyze the effect of the glycocalyx thickness on resistance to effector cell attack. Natural Killer (NK) cell-mediated cytotoxicity exhibits a near perfect inverse correlation with the glycocalyx thickness of target cells regardless of the specific glycan structures present, with changes in the glycocalyx thickness of approximately 10 nanometers significantly altering susceptibility to NK attack. Equipping the NK cell surface with a mucin-digesting enzyme can improve killing with a performance enhancement that can rival or exceed chimeric antigen receptors (CARs). Together, our results motivate the development of glycocalyx-editing immune cells for cancer immunotherapy.
-
Immunology and Microbiology
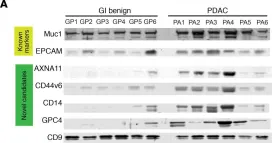
In JCI Insight on 5 November 2020 by Huang, L., Bockorny, B., et al.
Fig.8.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 1