Chemotherapy resistance in liver cancer is a major clinical issue, with CD147 playing a vital role in this process. However, the specific mechanisms underlying these processes remain largely unknown. This study investigates how CD147 internalization leads to cytoprotective autophagy, contributing to chemotherapy resistance in hepatocellular carcinoma (HCC).
Utilizing bioinformatics methods for KEGG pathways enrichment and screening key molecules associated with chemotherapy resistance through analyses of GEO and TCGA databases. An overexpression/knockdown system was used to study how CD147 internalization leads to autophagy in vitro and in vivo. The process was observed using microscopes, and molecular interactions and autophagy flux were analyzed. Analyzing the internalization of CD147 intracellular domains and the interaction with G3BP1 in clinical chemotherapy recurrence HCC tissues by immunohistochemistry, tissue immunofluorescence, and mass spectrometry. A tumor xenograft mice model was used to study cytoprotective autophagy induced by CD147 and test the effectiveness of combining cisplatin with an autophagy inhibitor in nude mice models.
In our study, we identified the tumor-associated membrane protein CD147, which implicated in chemoresistance lysosome pathways, by evaluating its protein degree value and betweenness centrality using Cytoscape. Our findings revealed that CD147 undergoes internalization and interacts with G3BP1 following treatment with cisplatin and methyl-β-cyclodextrin, forming a complex that is transported to lysosomes via Rab7A. Notably, higher doses of cisplatin enhanced CD147-mediated lysosomal transport while concurrently inhibiting SG assembly. The CD147-G3BP1 complex additionally inhibits mTOR activity, promoting autophagy and augmenting chemoresistance in hepatoma cells. In vivo studies investigations and analyses of clinical samples revealed that elevated internalization of CD147 is associated with chemotherapy recurrence in liver cancer and the maintenance of stem cells. Mice experiments found that the combined administration of cisplatin and hydroxychloroquine enhanced the efficacy of treatment.
This study reveals that CD147 internalization and CD147-G3BP1 complex translocation to lysosomes induce cytoprotective autophagy, reducing chemotherapy sensitivity by suppressing mTOR activity. It is also shown that chemotherapy drugs combined with autophagy inhibitors can improve the therapeutic effect of cancer, providing new insights into potential targeted therapeutic approaches in treating HCC.
© 2024. The Author(s).
Product Citations: 15
Targeting autophagy in HCC treatment: exploiting the CD147 internalization pathway.
In Cell Communication and Signaling : CCS on 3 December 2024 by Qian, M., Wan, Z., et al.
-
WB
-
Cell Biology
-
Endocrinology and Physiology
AC-73 and Syrosingopine Inhibit SARS-CoV-2 Entry into Megakaryocytes by Targeting CD147 and MCT4.
In Viruses on 4 January 2024 by Spinello, I., Saulle, E., et al.
Coagulation disorders are described in COVID-19 and long COVID patients. In particular, SARS-CoV-2 infection in megakaryocytes, which are precursors of platelets involved in thrombotic events in COVID-19, long COVID and, in rare cases, in vaccinated individuals, requires further investigation, particularly with the emergence of new SARS-CoV-2 variants. CD147, involved in the regulation of inflammation and required to fight virus infection, can facilitate SARS-CoV-2 entry into megakaryocytes. MCT4, a co-binding protein of CD147 and a key player in the glycolytic metabolism, could also play a role in SARS-CoV-2 infection. Here, we investigated the susceptibility of megakaryocytes to SARS-CoV-2 infection via CD147 and MCT4. We performed infection of Dami cells and human CD34+ hematopoietic progenitor cells induced to megakaryocytic differentiation with SARS-CoV-2 pseudovirus in the presence of AC-73 and syrosingopine, respective inhibitors of CD147 and MCT4 and inducers of autophagy, a process essential in megakaryocyte differentiation. Both AC-73 and syrosingopine enhance autophagy during differentiation but only AC-73 enhances megakaryocytic maturation. Importantly, we found that AC-73 or syrosingopine significantly inhibits SARS-CoV-2 infection of megakaryocytes. Altogether, our data indicate AC-73 and syrosingopine as inhibitors of SARS-CoV-2 infection via CD147 and MCT4 that can be used to prevent SARS-CoV-2 binding and entry into megakaryocytes, which are precursors of platelets involved in COVID-19-associated coagulopathy.
-
FC/FACS
-
COVID-19
Preprint on BioRxiv : the Preprint Server for Biology on 5 September 2023 by Hasegawa, H., Wang, S., et al.
Polymeric IgMs are secreted from plasma cells abundantly despite their structural complexity and intricate multimerization steps. To gain new insights into IgM’s assembly mechanics that underwrite the high-level secretion, we characterized the biosynthetic process of a natural human IgM, SAM-6, using a recombinant HEK293 cell system. By creating a series of mutant subunits that differentially disrupt specific sets of inter-chain disulfide bonds, we assessed their effects on various aspects of IgM biosynthesis in 48 different mutant subunit combinations. The analysis included the visualization of intracellular biosynthetic events such as steady-state subcellular subunit distribution, secretory trafficking bottlenecks, and the ER-associated Russell body formation by fluorescent microscopy. We also characterized various extracellular events including secreted IgM product quality, secretion output, and the release of various assembly intermediates using biochemical and biophysical assays. In this combinatorial mutagenesis approach, we unexpectedly found that the loss of multiple inter-chain disulfide bonds, including the one between μHC and λLC subunits, was tolerated in polymeric IgM formation and secretion. This finding revealed the vital role of underlying non-covalent protein-protein association not only during the orchestration of initial subunit interactions but also in maintaining the polymeric IgM product integrity during ER quality control steps, secretory pathway trafficking, and secretion. We suggest that the IgM assembly process is inherently robust and has a stopgap that permits the secretion of polymeric IgM even when not all the prescribed inter-chain disulfide bonds are formed. This study holistically presents the requirements and exemptions in polymeric IgM biosynthesis by encompassing the characterization of intracellular and extracellular events and the roles of covalent and non-covalent interactions. These findings can guide antibody engineering strategy when designing IgM-based multivalent modalities.
-
Homo sapiens (Human)
In Nature Communications on 23 February 2021 by Askarian, F., Uchiyama, S., et al.
The recently discovered lytic polysaccharide monooxygenases (LPMOs), which cleave polysaccharides by oxidation, have been associated with bacterial virulence, but supporting functional data is scarce. Here we show that CbpD, the LPMO of Pseudomonas aeruginosa, is a chitin-oxidizing virulence factor that promotes survival of the bacterium in human blood. The catalytic activity of CbpD was promoted by azurin and pyocyanin, two redox-active virulence factors also secreted by P. aeruginosa. Homology modeling, molecular dynamics simulations, and small angle X-ray scattering indicated that CbpD is a monomeric tri-modular enzyme with flexible linkers. Deletion of cbpD rendered P. aeruginosa unable to establish a lethal systemic infection, associated with enhanced bacterial clearance in vivo. CbpD-dependent survival of the wild-type bacterium was not attributable to dampening of pro-inflammatory responses by CbpD ex vivo or in vivo. Rather, we found that CbpD attenuates the terminal complement cascade in human serum. Studies with an active site mutant of CbpD indicated that catalytic activity is crucial for virulence function. Finally, profiling of the bacterial and splenic proteomes showed that the lack of this single enzyme resulted in substantial re-organization of the bacterial and host proteomes. LPMOs similar to CbpD occur in other pathogens and may have similar immune evasive functions.
-
FC/FACS
-
Immunology and Microbiology
Identification of ADAM12 as a Novel Basigin Sheddase.
In International Journal of Molecular Sciences on 22 April 2019 by Albrechtsen, R., Wewer Albrechtsen, N. J., et al.
The transmembrane glycoprotein basigin, a member of the immunoglobulin superfamily, stimulates matrix metalloproteinase (MMP)-mediated extracellular matrix (ECM) degradation and thereby drives cancer cell invasion. Basigin is proteolytically shed from the cell surface and high concentrations of soluble basigin in the blood dictates poor prognosis in cancer patients. A positive correlation between basigin and a disintegrin and metalloproteinase (ADAM)-12 in serum from prostate cancer patients has been reported. Yet, the functional relevance of this correlation is unknown. Here, we show that ADAM12 interacts with basigin and cleaves it in the juxtamembrane region. Specifically, overexpression of ADAM12 increases ectodomain shedding of an alkaline phosphatase-tagged basigin reporter protein from the cell surface. Moreover, CRISPR/Cas9-mediated knockout of ADAM12 in human HeLa carcinoma cells results in reduced shedding of the basigin reporter, which can be rescued by ADAM12 re-expression. We detected endogenous basigin fragments, corresponding to the expected size of the ADAM12-generated ectodomain, in conditioned media from ADAM12 expressing cancer cell-lines, as well as serum samples from a healthy pregnant donor and five bladder cancer patients, known to contain high ADAM12 levels. Supporting the cancer relevance of our findings, we identified several cancer-associated mutations in the basigin membrane proximal region. Subsequent in vitro expression showed that some of these mutants are more prone to ADAM12-mediated shedding and that the shed ectodomain can enhance gelatin degradation by cancer cells. In conclusion, we identified ADAM12 as a novel basigin sheddase with a potential implication in cancer.
-
WB post IP
-
Homo sapiens (Human)
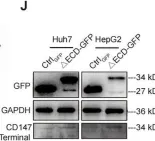
In Cell Commun Signal on 3 December 2024 by Qian, M., Wan, Z., et al.
Fig.2.J

-
WB
-
Collected and cropped from Cell Commun Signal by CiteAb, provided under a CC-BY license
Image 1 of 3
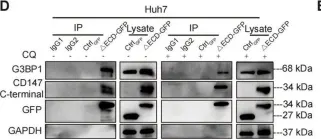
In Cell Commun Signal on 3 December 2024 by Qian, M., Wan, Z., et al.
Fig.3.D

-
WB
-
Collected and cropped from Cell Commun Signal by CiteAb, provided under a CC-BY license
Image 1 of 3
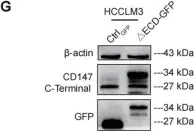
In Cell Commun Signal on 3 December 2024 by Qian, M., Wan, Z., et al.
Fig.5.G

-
WB
-
Collected and cropped from Cell Commun Signal by CiteAb, provided under a CC-BY license
Image 1 of 3